Multiple myeloma induces Mcl-1 expression and survival of myeloid-derived suppressor cells
- PMID: 25871384
- PMCID: PMC4496373
- DOI: 10.18632/oncotarget.3300
Multiple myeloma induces Mcl-1 expression and survival of myeloid-derived suppressor cells
Abstract
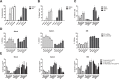
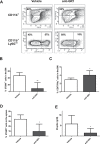
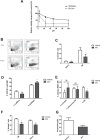
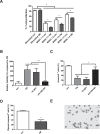
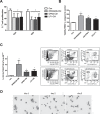
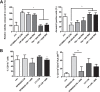
Myeloid-derived suppressor cells (MDSC) are contributing to an immunosuppressive environment by their ability to inhibit T cell activity and thereby promoting cancer progression. An important feature of the incurable plasma cell malignancy Multiple Myeloma (MM) is immune dysfunction. MDSC were previously identified to be present and active in MM patients, however little is known about the MDSC-inducing and -activating capacity of MM cells. In this study we investigated the effects of the tumor microenvironment on MDSC survival. During MM progression in the 5TMM mouse model, accumulation of MDSC in the bone marrow was observed in early stages of disease development, while circulating myeloid cells were increased at later stages of disease. Interestingly, in vivo MDSC targeting by anti-GR1 antibodies and 5-Fluorouracil resulted in a significant reduced tumor load in 5TMM-diseased mice. In vitro generation of MDSC was demonstrated by increased T cell immunosuppressive capacity and MDSC survival was observed in the presence of MM-conditioned medium. Finally, increased Mcl-1 expression was identified as underlying mechanism for MDSC survival. In conclusion, our data demonstrate that soluble factors from MM cells are able to generate MDSC through Mcl-1 upregulation and this cell population can be considered as a possible target in MM disease.
Keywords: generation; mechanism; multiple myeloma; myeloid-derived suppressor cells; targeting.
Conflict of interest statement
The authors disclose no potential conflicts of interest.
Figures

References
-
- Kyle R a, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351:1860–73. - PubMed
-
- Nur H, Van Valckenborgh E, De Bruyne E, Vanderkerken K, Menu E. The Role of Invariant Natural Killer T Cells in Cancer. Int Blood Res Rev. 2014;1:44–71.
-
- Pan B, Lentzsch S. The application and biology of immunomodulatory drugs (IMiDs) in cancer. Pharmacol Ther. 2012;136:56–68. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

