Co-inhibition of polo-like kinase 1 and Aurora kinases promotes mitotic catastrophe
- PMID: 25871386
- PMCID: PMC4496220
- DOI: 10.18632/oncotarget.3313
Co-inhibition of polo-like kinase 1 and Aurora kinases promotes mitotic catastrophe
Abstract
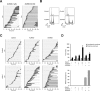
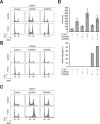
Mitosis is choreographed by a number of protein kinases including polo-like kinases and Aurora kinases. As these kinases are frequently dysregulated in cancers, small-molecule inhibitors have been developed for targeted anticancer therapies. Given that PLK1 and Aurora kinases possess both unique functions as well as co-regulate multiple mitotic events, whether pharmacological inhibition of these kinases together can enhance mitotic catastrophe remains an outstanding issue to be determined. Using concentrations of inhibitors that did not induce severe mitotic defects on their own, we found that both the metaphase arrest and mitotic slippage induced by inhibitors targeting Aurora A and Aurora B (MK-5108 and Barasertib respectively) were enhanced by a PLK1 inhibitor (BI 2536). We found that PLK1 is overexpressed in cells from nasopharyngeal carcinoma, a highly invasive cancer with poor prognosis, in comparison to normal nasopharyngeal epithelial cells. Nasopharyngeal carcinoma cells were more sensitive to BI 2536 as a single agent and co-inhibition with Aurora kinases than normal cells. These observations underscore the mechanism and potential benefits of targeting PLK1 and Aurora kinases to induce mitotic catastrophe in cancer cells.
Keywords: anticancer drugs; antimitotic drugs; kinases; mitosis; mitotic slippage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Ma HT, Poon RY. How protein kinases co-ordinate mitosis in animal cells. Biochem J. 2011;435:17–31. - PubMed
-
- Fung TK, Poon RY. A roller coaster ride with the mitotic cyclins. Semin Cell Dev Biol. 2005;16:335–42. - PubMed
-
- Lobjois V, Jullien D, Bouche JP, Ducommun B. The polo-like kinase 1 regulates CDC25B-dependent mitosis entry. Biochim Biophys Acta. 2009;1793:462–8. - PubMed
-
- Roshak AK, Capper EA, Imburgia C, Fornwald J, Scott G, Marshall LA. The human polo-like kinase, PLK, regulates cdc2/cyclin B through phosphorylation and activation of the cdc25C phosphatase. Cell Signal. 2000;12:405–11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

