Circadian Control of the Female Reproductive Axis Through Gated Responsiveness of the RFRP-3 System to VIP Signaling
- PMID: 25872006
- PMCID: PMC4475714
- DOI: 10.1210/en.2014-1762
Circadian Control of the Female Reproductive Axis Through Gated Responsiveness of the RFRP-3 System to VIP Signaling
Abstract
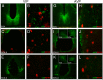
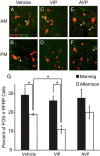
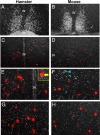
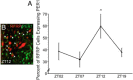
Throughout most of the ovulatory cycle, estrogen negative feedback restrains the GnRH neuronal system. Just before ovulation, however, estrogen negative feedback is removed to permit stimulation of the preovulatory GnRH/LH surge (positive feedback) by the circadian clock in the suprachiasmatic nucleus (SCN). The mammalian ortholog of avian gonadotropin-inhibitory hormone, RFamide-related peptide 3 (RFRP-3), participates in the circadian-timed removal of estrogen negative feedback to permit the LH surge. The present study examined the specific neurochemical means by which the SCN controls RFRP-3 activity and explored whether the RFRP-3 system exhibits time-dependent responsiveness to SCN signaling to precisely time the LH surge. We found that RFRP-3 cells in female Syrian hamsters (Mesocricetus auratus) receive close appositions from SCN-derived vasopressin-ergic and vasoactive intestinal peptide (VIP)-ergic terminal fibers. Central VIP administration markedly suppressed RFRP-3 cellular activity in the evening, but not the morning, relative to saline controls, whereas vasopressin was without effect at either time point. Double-label in situ hybridization for Rfrp-3 and the VIP receptors VPAC1 and VPAC2 revealed that the majority of RFRP-3 cells do not coexpress either receptor in Syrian hamsters or mice, suggesting that SCN VIP-ergic signaling inhibits RFRP-3 cells indirectly. The timing of this VIP-mediated disinhibition is further coordinated via temporally gated responsiveness of RFRP-3 cells to circadian signaling. Together, these findings reveal a novel circadian hierarchy of control coordinating the preovulatory LH surge and ovulation.
Figures


References
-
- Ahlborg G, Jr, Axelsson G, Bodin L. Shift work, nitrous oxide exposure and subfertility among Swedish midwives. Int J Epidemiol. 1996;25(4):783–790. - PubMed
-
- Bisanti L, Olsen J, Basso O, Thonneau P, Karmaus W. Shift work and subfecundity: a European multicenter study. European Study Group on Infertility and Subfecundity. J Occup Environ Med. 1996;38(4):352–358. - PubMed
-
- Nurminen T. Shift work and reproductive health. Scand J Work Environ Health. 1998;24(suppl 3):28–34. - PubMed
-
- Endo A, Watanabe T. Effects of non-24-hour days on reproductive efficacy and embryonic development in mice. Gamete Res. 1989;22(4):435–441. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

