From ELF to compressibility in solids
- PMID: 25872139
- PMCID: PMC4425073
- DOI: 10.3390/ijms16048151
From ELF to compressibility in solids
Abstract
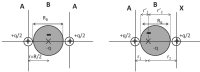
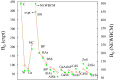
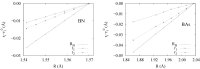
Understanding the electronic nature of materials' compressibility has always been a major issue behind tabulation and rationalization of bulk moduli. This is especially because this understanding is one of the main approaches to the design and proposal of new materials with a desired (e.g., ultralow) compressibility. It is well recognized that the softest part of the solid will be the one responsible for its compression at the first place. In chemical terms, this means that the valence will suffer the main consequences of pressurization.It is desirable to understand this response to pressure in terms of the valence properties(charge, volume, etc.). One of the possible approaches is to consider models of electronic separability, such as the bond charge model (BCM), which provides insight into the cohesion of covalent crystals in analogy with the classical ionic model. However, this model relies on empirical parametrization of bond and lone pair properties. In this contribution, we have coupled electron localization function (ELF) ab initio data with the bond charge model developed by Parr in order to analyze solid state compressibility from first principles and moreover, to derive general trends and shed light upon superhard behavior.
Figures


References
-
- Gilman J.J. Electronic Basis of the Strength of Materials. Cambridge University Press; Cambridge, UK: 2003.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

