SMS-based smoking cessation intervention among university students: study protocol for a randomised controlled trial (NEXit trial)
- PMID: 25872503
- PMCID: PMC4403894
- DOI: 10.1186/s13063-015-0640-2
SMS-based smoking cessation intervention among university students: study protocol for a randomised controlled trial (NEXit trial)
Abstract
Background: Most smoking efforts targeting young people have so far been focused on prevention of initiation, whereas smoking cessation interventions have largely been targeted towards adult populations. Thus, there is limited evidence for effective smoking cessation interventions in young people, even though many young people want to quit smoking. Mobile communication technology has the potential to reach large numbers of young people and recent text-based smoking cessation interventions using phones have shown promising results.
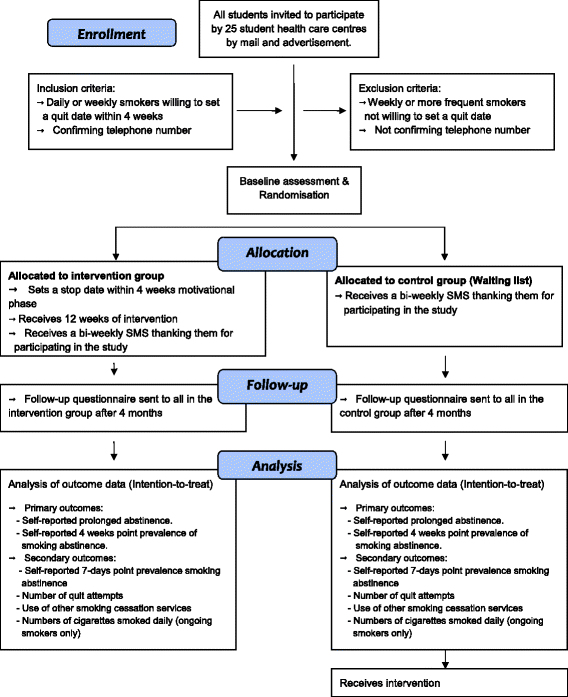
Methods/design: The study aims to evaluate a newly developed text-based smoking cessation intervention for students in colleges and universities in Sweden. The design is a randomised controlled trial (RCT) with a delayed/waiting list intervention control condition. The trial will be performed simultaneously in all colleges and universities served by 25 student health care centres in Sweden. Outcomes will be evaluated after 4 months, with 2 cessation primary outcomes and 4 secondary outcomes. After outcome evaluation the control group will be given access to the intervention.
Discussion: The study will examine the effectiveness of a stand-alone SMS text-based intervention. The intervention starts with a motivational phase in which the participants are given an opportunity to set a quit date within 4 weeks of randomisation. This first phase and the subsequent core intervention phase of 12 weeks are totally automated in order to easily integrate the intervention into the daily routines of student and other health care settings. As well as providing data for the effectiveness of the intervention, the study will also provide data for methodological analyses addressing a number issues commonly challenging in Internet-based RCTs. For example, an extensive follow-up strategy will be used in order to evaluate the use of repeated attempts in the analysis, and in particular to explore the validity of a possible missing not at random assumption that the odds ratio between the primary outcome and response is the same at every attempt.
Isrctn: ISRCTN75766527, dated assigned 4 November 2014. Protocol version: Version 1, and date 7 November 2014.
Figures
Similar articles
-
Effectiveness of Short Message Service Text-Based Smoking Cessation Intervention Among University Students: A Randomized Clinical Trial.JAMA Intern Med. 2016 Mar;176(3):321-8. doi: 10.1001/jamainternmed.2015.8260. JAMA Intern Med. 2016. PMID: 26903176 Free PMC article. Clinical Trial.
-
User satisfaction with the structure and content of the NEXit intervention, a text messaging-based smoking cessation programme.BMC Public Health. 2016 Nov 22;16(1):1179. doi: 10.1186/s12889-016-3848-5. BMC Public Health. 2016. PMID: 27876031 Free PMC article. Clinical Trial.
-
Efficacy of a text messaging (SMS) based smoking cessation intervention for adolescents and young adults: study protocol of a cluster randomised controlled trial.BMC Public Health. 2012 Jan 19;12:51. doi: 10.1186/1471-2458-12-51. BMC Public Health. 2012. PMID: 22260736 Free PMC article. Clinical Trial.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Interventions to reduce tobacco use in people experiencing homelessness.Cochrane Database Syst Rev. 2020 Dec 3;12(12):CD013413. doi: 10.1002/14651858.CD013413.pub2. Cochrane Database Syst Rev. 2020. PMID: 33284989 Free PMC article.
Cited by
-
Implementing Facilitated Access to a Text Messaging, Smoking Cessation Intervention Among Swedish Patients Having Elective Surgery: Qualitative Study of Patients' and Health Care Professionals' Perspectives.JMIR Mhealth Uhealth. 2020 Sep 18;8(9):e17563. doi: 10.2196/17563. JMIR Mhealth Uhealth. 2020. PMID: 32945772 Free PMC article.
-
Exploring the Experiences of Individuals Allocated to a Control Setting: Findings From a Mobile Health Smoking Cessation Trial.JMIR Hum Factors. 2019 Apr 2;6(2):e12139. doi: 10.2196/12139. JMIR Hum Factors. 2019. PMID: 30938682 Free PMC article.
-
Effectiveness of Short Message Service Text-Based Smoking Cessation Intervention Among University Students: A Randomized Clinical Trial.JAMA Intern Med. 2016 Mar;176(3):321-8. doi: 10.1001/jamainternmed.2015.8260. JAMA Intern Med. 2016. PMID: 26903176 Free PMC article. Clinical Trial.
-
mHealth smoking cessation intervention among high-school pupils (NEXit Junior): study protocol for a randomized controlled trial.Trials. 2018 Nov 22;19(1):648. doi: 10.1186/s13063-018-3028-2. Trials. 2018. PMID: 30466484 Free PMC article.
-
Smart Technology in Lung Disease Clinical Trials.Chest. 2016 Jan;149(1):22-6. doi: 10.1378/chest.15-1314. Epub 2016 Jan 6. Chest. 2016. PMID: 26135330 Free PMC article.
References
-
- Gilliam H. Quitting smoking brings quick health benefits. Lakartidningen. 2012;109:554–7. - PubMed
-
- The Public Health Agency of Sweden. http://www.folkhalsomyndigheten.se/amnesomraden/statistik-och-undersokni.... Last accessed 2 October 2014.
-
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–60. doi: 10.1016/S0140-6736(12)61766-8. - DOI - PMC - PubMed
-
- Agardh E, Moradi T, Allebeck P. The contribution of risk factors to the burden of disease in Sweden. A comparison between Swedish and WHO data. Lakartidningen. 2008;105:816–21. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials