Altered Corticostriatal Connectivity and Exploration/Exploitation Imbalance Emerge as Intermediate Phenotypes for a Neonatal Dopamine Dysfunction
- PMID: 25872916
- PMCID: PMC4569947
- DOI: 10.1038/npp.2015.104
Altered Corticostriatal Connectivity and Exploration/Exploitation Imbalance Emerge as Intermediate Phenotypes for a Neonatal Dopamine Dysfunction
Abstract
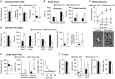
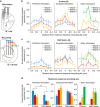
Findings showing that neonatal lesions of the forebrain dopaminergic system in rodents lead to juvenile locomotor hyperactivity and learning deficits have been taken as evidence of face validity for the attention deficit hyperactivity disorder. However, the core cognitive and physiological intermediate phenotypes underlying this rodent syndrome remain unknown. Here we show that early postnatal dopaminergic lesions cause long-lasting deficits in exploitation of shelter, social and nutritional resources, and an imbalanced exploratory behavior, where nondirected local exploration is exacerbated, whereas sophisticated search behaviors involving sequences of goal directed actions are degraded. Importantly, some behavioral deficits do not diminish after adolescence but instead worsen or mutate, particularly those related to the exploration of wide and spatially complex environments. The in vivo electrophysiological recordings and morphological reconstructions of striatal medium spiny neurons reveal corticostriatal alterations associated to the behavioral phenotype. More specifically, an attenuation of corticostriatal functional connectivity, affecting medial prefrontal inputs more markedly than cingulate and motor inputs, is accompanied by a contraction of the dendritic arbor of striatal projection neurons in this animal model. Thus, dopaminergic neurons are essential during postnatal development for the functional and structural maturation of corticostriatal connections. From a bottom-up viewpoint, our findings suggest that neuropsychiatric conditions presumably linked to developmental alterations of the dopaminergic system should be evaluated for deficits in foraging decision making, alterations in the recruitment of corticostriatal circuits during foraging tasks, and structural disorganization of the frontostriatal connections.
Figures




References
-
- Archer T, Danysz W, Fredriksson A, Jonsson G, Luthman J, Sundström E, et al. Neonatal 6-hydroxydopamine-induced dopamine depletions: motor activity and performance in maze learning. Pharmacol Biochem Behav. 1988;31:357–364. - PubMed
-
- Avale ME, Falzone TL, Gelman DM, Low MJ, Grandy DK, Rubinstein M. The dopamine D4 receptor is essential for hyperactivity and impaired behavioral inhibition in a mouse model of attention deficit/hyperactivity disorder. Mol Psychiatry. 2004;9:718–726. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

