Evaluation of tumour vaccine immunotherapy for the treatment of advanced non-small cell lung cancer: a systematic meta-analysis
- PMID: 25872936
- PMCID: PMC4401843
- DOI: 10.1136/bmjopen-2014-006321
Evaluation of tumour vaccine immunotherapy for the treatment of advanced non-small cell lung cancer: a systematic meta-analysis
Abstract
Objectives: Our meta-analysis performed a systematic evaluation on the therapeutic efficacy and safety of tumour vaccines for the treatment of advanced non-small cell lung cancer (NSCLC).
Design: Systematic review and meta-analysis of randomised controlled trials (RCT).
Data sources: PubMed, the Cochrane Center Register of Controlled Trials, Science Direct and EMBASE were searched from January 1980 until January 2015.
Eligibility criteria for selecting studies: RCT were included; the control arm had to receive either placebo or chemotherapy or no treatment.
Main outcome measures: The quality of the data from individual papers was assessed for overall survival (OS), clinical response rate and side effects.
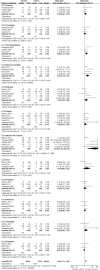
Results: Overall, 11 RCT of advanced NSCLC with a total of 3986 patients were conducted for meta-analysis. The results showed that the vaccine arm significantly extended primary endpoint median overall survival compared with control group (p<0.00001) (HR 0.760; 95% CI 0.644 to 0.896; p=0.001). Three subgroup patients with tumour vaccine at 1-year, 2-year and 3-year survival rates also gained significant benefits compared with their corresponding control group (p=0.0004, 0.03 and 0.19, respectively). Besides, a significant improvement in median time to progression (TTP), median progression-free survival (PFS) and a trend of improvement in objective response rate were observed after tumour vaccine treatment (p=0.001, 0.005 and 0.05, respectively; median PFS HR 0.842; 95% CI 0.744 to 0.954; p=0.007). A few severe adverse effects occurred in the tumour vaccine group, but fewer side effects were observed in the vaccine group compared with the control group (p<0.00001).
Conclusions: Taken together, NSCLC tumour vaccines markedly prolong median OS (p<0.00001), median TTP (p=0.001) and median PFS (p=0.005), improve clinical response rate (p=0.05) and lessen adverse side effects (p<0.00001). Our meta-analysis suggests tumour vaccines improve the efficacy of the treatment, and also provide superiority in treatment of patients with advanced NSCLC among a variety of immunotherapy strategies.
Keywords: Immunotherapy; Non-small cell lung cancer; Tumor vaccine.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures


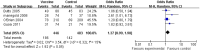

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
