Donor CD4 T Cell Diversity Determines Virus Reactivation in Patients After HLA-Matched Allogeneic Stem Cell Transplantation
- PMID: 25873100
- PMCID: PMC4654256
- DOI: 10.1111/ajt.13241
Donor CD4 T Cell Diversity Determines Virus Reactivation in Patients After HLA-Matched Allogeneic Stem Cell Transplantation
Abstract
Delayed reconstitution of the T cell compartment in recipients of allogeneic stem cell grafts is associated with an increase of reactivation of latent viruses. Thereby, the transplanted T cell repertoire appears to be one of the factors that affect T cell reconstitution. Therefore, we studied the T cell receptor beta (TCRβ) gene rearrangements of flow cytometry-sorted CD4(+) and CD8(+) T cells from the peripheral blood of 23 allogeneic donors before G-CSF administration and on the day of apheresis. For this purpose, TCRβ rearrangements were amplified by multiplex PCR followed by high-throughput amplicon sequencing. Overall, CD4(+) T cells displayed a significantly higher TCRβ diversity compared to CD8(+) T cells irrespective of G-CSF administration. In line, no significant impact of G-CSF treatment on the TCR Vβ repertoire usage was found. However, correlation of the donor T cell repertoire with clinical outcomes of the recipient revealed that a higher CD4(+) TCRβ diversity after G-CSF treatment is associated with lower reactivation of cytomegalovirus and Epstein-Barr virus. By contrast, no protecting correlation was observed for CD8(+) T cells. In essence, our deep TCRβ analysis identifies the importance of the CD4(+) T cell compartment for the control of latent viruses after allogeneic stem cell transplantation.
Keywords: Bone marrow; Epstein-Barr virus (EBV); T cell biology; cytomegalovirus (CMV); donors and donation; hematopoietic stem cell transplantation; infection and infectious agents; infectious disease; molecular biology; science; translational research.
© 2015 The Authors. American Journal of Transplantation Published by Wiley Periodicals, Inc.
Figures


 CD4+ T cells preMOB;
CD4+ T cells preMOB;  CD4+ T cells postMOB;
CD4+ T cells postMOB;  CD8+ T cells preMOB;
CD8+ T cells preMOB;  CD8+ T cells postMOB.
CD8+ T cells postMOB.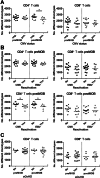
 CD4+ T cells preMOB;
CD4+ T cells preMOB;  CD4+ T cells postMOB;
CD4+ T cells postMOB;  CD8+ T cells preMOB;
CD8+ T cells preMOB;  CD8+ T cells postMOB.
CD8+ T cells postMOB.References
-
- Theilgaard-Monch K, Raaschou-Jensen K, Andersen H, et al. Single leukapheresis products collected from healthy donors after the administration of granulocyte colony-stimulating factor contain ten-fold higher numbers of long-term reconstituting hematopoietic progenitor cells than conventional bone marrow allografts. Bone Marrow Transplant. 1999;3:243–249. - PubMed
-
- Pan L, Delmonte J, Jr, Jalonen CK, Ferrara JL. Pretreatment of donor mice with granulocyte colony-stimulating factor polarizes donor T lymphocytes toward type-2 cytokine production and reduces severity of experimental graft-versus-host disease. Blood. 1995;12:4422–4429. - PubMed
-
- Seggewiss R, Einsele H. Immune reconstitution after allogeneic transplantation and expanding options for immunomodulation: An update. Blood. 2010;19:3861–3868. - PubMed
-
- Fallen PR, McGreavey L, Madrigal JA, et al. Factors affecting reconstitution of the T cell compartment in allogeneic haematopoietic cell transplant recipients. Bone Marrow Transplant. 2003;10:1001–1014. - PubMed
-
- Maury S, Mary JY, Rabian C, et al. Prolonged immune deficiency following allogeneic stem cell transplantation: Risk factors and complications in adult patients. Br J Haematol. 2001;3:630–641. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

