Cell Injury-Induced Release of Fibroblast Growth Factor 2: Relevance to Intracerebral Mesenchymal Stromal Cell Transplantations
- PMID: 25873141
- PMCID: PMC4499789
- DOI: 10.1089/scd.2015.0083
Cell Injury-Induced Release of Fibroblast Growth Factor 2: Relevance to Intracerebral Mesenchymal Stromal Cell Transplantations
Abstract
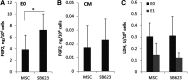
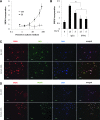
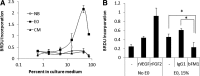
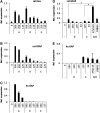
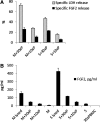
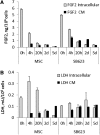
Beneficial effects of intracerebral transplantation of mesenchymal stromal cells (MSC) and their derivatives are believed to be mediated mostly by factors produced by engrafted cells. However, the mesenchymal cell engraftment rate is low, and the majority of grafted cells disappear within a short post-transplantation period. Here, we hypothesize that dying transplanted cells can affect surrounding tissues by releasing their active intracellular components. To elucidate the type, amounts, and potency of these putative intracellular factors, freeze/thaw extracts of MSC or their derivatives were tested in enzyme-linked immunosorbent assays and bioassays. We found that fibroblast growth factor (FGF)2 and FGF1, but not vascular endothelial growth factor and monocyte chemoattractant protein 1 levels were high in extracts despite being low in conditioned media. Extracts induced concentration-dependent proliferation of rat cortical neural progenitor cells and human umbilical vein endothelial cells; these proliferative responses were specifically blocked by FGF2-neutralizing antibody. In the neuropoiesis assay with rat cortical cells, both MSC extracts and killed cells induced expression of nestin, but not astrocyte differentiation. However, suspensions of killed cells strongly potentiated the astrogenic effects of live MSC. In transplantation-relevant MSC injury models (peripheral blood cell-mediated cytotoxicity and high cell density plating), MSC death coincided with the release of intracellular FGF2. The data showed that MSC contain a major depot of active FGF2 that is released upon cell injury and is capable of acutely stimulating neuropoiesis and angiogenesis. We therefore propose that both dying and surviving grafted MSC contribute to tissue regeneration.
Figures
Similar articles
-
Comparison of the neuropoietic activity of gene-modified versus parental mesenchymal stromal cells and the identification of soluble and extracellular matrix-related neuropoietic mediators.Stem Cell Res Ther. 2014 Feb 26;5(1):29. doi: 10.1186/scrt418. Stem Cell Res Ther. 2014. PMID: 24572070 Free PMC article.
-
Quantitative microplate assay for studying mesenchymal stromal cell-induced neuropoiesis.Stem Cells Transl Med. 2013 Mar;2(3):223-32. doi: 10.5966/sctm.2012-0119. Epub 2013 Feb 19. Stem Cells Transl Med. 2013. PMID: 23430693 Free PMC article.
-
Mesenchymal stem cell secreted platelet derived growth factor exerts a pro-migratory effect on resident Cardiac Atrial appendage Stem Cells.J Mol Cell Cardiol. 2014 Jan;66:177-88. doi: 10.1016/j.yjmcc.2013.11.016. Epub 2013 Dec 8. J Mol Cell Cardiol. 2014. PMID: 24326234
-
[Mesenchymal stromal progenitor cells: general characteristics and functional state in low oxygen tension].Ross Fiziol Zh Im I M Sechenova. 2008 Jul;94(7):737-57. Ross Fiziol Zh Im I M Sechenova. 2008. PMID: 18767387 Review. Russian.
-
[Basic science and clinical applications of mesenchymal stem cells].Seikagaku. 2009 Feb;81(2):99-104. Seikagaku. 2009. PMID: 19306655 Review. Japanese. No abstract available.
Cited by
-
Dry preserved multilayered fibroblast cell sheets are a new manageable tool for regenerative medicine to promote wound healing.Sci Rep. 2022 Jul 22;12(1):12519. doi: 10.1038/s41598-022-16345-6. Sci Rep. 2022. PMID: 35869108 Free PMC article.
-
Therapeutic implications of how TNF links apolipoprotein E, phosphorylated tau, α-synuclein, amyloid-β and insulin resistance in neurodegenerative diseases.Br J Pharmacol. 2018 Oct;175(20):3859-3875. doi: 10.1111/bph.14471. Epub 2018 Sep 6. Br J Pharmacol. 2018. PMID: 30097997 Free PMC article. Review.
-
Mesenchymal Stem Cell Mechanisms of Action and Clinical Effects in Osteoarthritis: A Narrative Review.Genes (Basel). 2022 May 26;13(6):949. doi: 10.3390/genes13060949. Genes (Basel). 2022. PMID: 35741711 Free PMC article. Review.
-
Acceleration of Fracture Healing by Overexpression of Basic Fibroblast Growth Factor in the Mesenchymal Stromal Cells.Stem Cells Transl Med. 2017 Oct;6(10):1880-1893. doi: 10.1002/sctm.17-0039. Epub 2017 Aug 9. Stem Cells Transl Med. 2017. PMID: 28792122 Free PMC article.
-
Fibroblasts are the most suitable cell source for regenerative medicine due to their high intracellular fibroblast growth factor 2 content.Biochem Biophys Rep. 2023 Jul 5;35:101510. doi: 10.1016/j.bbrep.2023.101510. eCollection 2023 Sep. Biochem Biophys Rep. 2023. PMID: 37457362 Free PMC article.
References
-
- Caplan AI. and Dennis JE. (2006). Mesenchymal stem cells as trophic mediators. J Cell Biochem 98:1076–1084 - PubMed
-
- Bang OY, Lee JS. and Lee PH. (2005). Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol 57:874–882 - PubMed
-
- Isakova IA, Baker K, Dufour J, Gaupp D. and Phinney DG. (2006). Preclinical evaluation of adult stem cell engraftment and toxicity in the CNS of rhesus macaques. Mol Ther 3:1173–1184 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
