Comparing different dosing regimens of bevacizumab in the treatment of neovascular macular degeneration: study protocol for a randomised controlled trial
- PMID: 25873213
- PMCID: PMC4376508
- DOI: 10.1186/s13063-015-0608-2
Comparing different dosing regimens of bevacizumab in the treatment of neovascular macular degeneration: study protocol for a randomised controlled trial
Abstract
Background: Bevacizumab (Avastin®) is as effective as ranibizumab (Lucentis®) in the treatment of neovascular age-related macular degeneration (nAMD). However it has two important structural differences. First, it has two active sites instead of one; second, it retains the Fc portion of the antibody which would be expected to confer a significantly longer half-life. These agents have been associated with systemic complications including strokes, so it is desirable to use the smallest effective dose. Furthermore, the standard dosing regimen requires monthly hospital visits, which present a significant challenge both to the hospital services and to the patients (who are elderly).
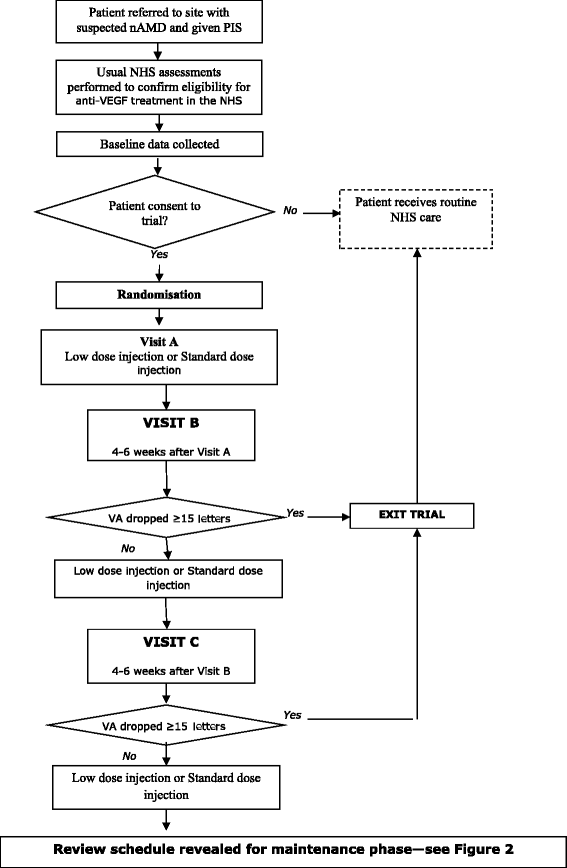
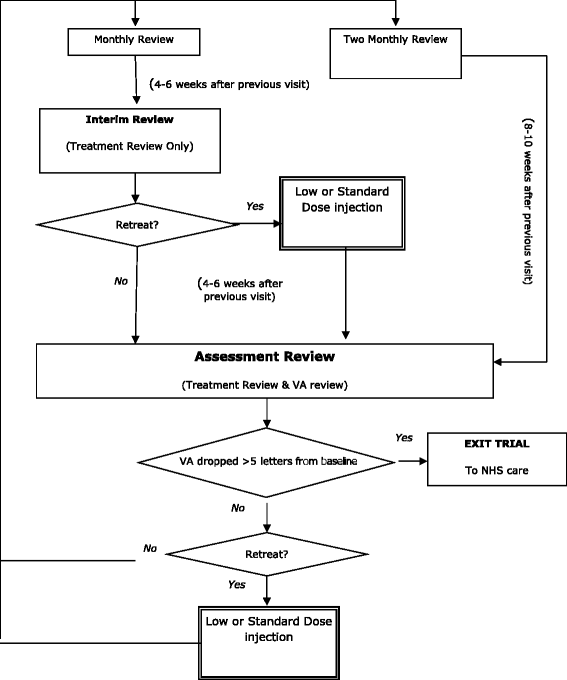
Methods/design: Patients ≥50 years who are eligible for anti-vascular endothelial growth factor (VEGF) treatment of nAMD in the NHS, who are either newly referred for treatment or have reactivation of nAMD and who have not received treatment to either eye for the previous six months. We have designed a factorial multi-centre masked randomised controlled trial using bevacizumab as the intervention, with patients randomised to one of four arms: to standard or low dose and to monthly or two-monthly patient review. The aim is to recruit sufficient patients (around 1,000) to obtain 304 patients meeting the endpoint over a four-year period. The primary endpoint is time to treatment failure to be analysed using Cox regression.
Discussion: This randomised control trial will show if half dose and two monthly as required is as effective as full dose and monthly regimes. A two monthly as required regimen of Bevacizumab would significantly reduce both the cost and the service delivery burden for the treatment of nAMD while a reduced dose would be expected to enhance the safety profile of this treatment regime.
Trial registration: International Standard Randomised Controlled Trial Number: ISRCTN95654194 , registered on 22 September 2009.
Figures
References
-
- Royal College of Ophthalmologists . Age-Related Macular Degeneration. Guidelines for Management. London: Royal College of Ophthalmologists; 2009.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical