The effects of interfacial potential on antimicrobial propensity of ZnO nanoparticle
- PMID: 25873247
- PMCID: PMC4397836
- DOI: 10.1038/srep09578
The effects of interfacial potential on antimicrobial propensity of ZnO nanoparticle
Abstract
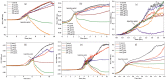
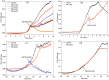
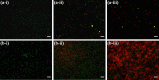
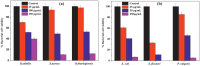
The work investigates the role of interfacial potential in defining antimicrobial propensity of ZnO nanoparticle (ZnONP) against different Gram positive and Gram negative bacteria. ZnONPs with positive and negative surface potential are tested against different bacteria with varying surface potentials, ranging -14.7 to -23.6 mV. Chemically synthesized ZnONPs with positive surface potential show very high antimicrobial propensity with minimum inhibitory concentration of 50 and 100 μg/mL for Gram negative and positive bacterium, respectively. On other hand, ZnONPs of the same size but with negative surface potential show insignificant antimicrobial propensity against the studied bacteria. Unlike the positively charged nanoparticles, neither Zn(2+) ion nor negatively charged ZnONP shows any significant inhibition in growth or morphology of the bacterium. Potential neutralization and colony forming unit studies together proved adverse effect of the resultant nano-bacterial interfacial potential on bacterial viability. Thus, ZnONP with positive surface potential upon interaction with negative surface potential of bacterial membrane enhances production of the reactive oxygen species and exerts mechanical stress on the membrane, resulting in the membrane depolarization. Our results show that the antimicrobial propensity of metal oxide nanoparticle mainly depends upon the interfacial potential, the potential resulting upon interaction of nanoparticle surface with bacterial membrane.
Figures





References
-
- Nel A. E. et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 8, 543–557 (2009). - PubMed
-
- Dewan S. et al. Structure of water at charged interfaces: a molecular dynamics study. Langmuir 30, 8056–8065 (2014). - PubMed
-
- Monopoli M. P., berg C., Salvati A. & Dawson K. A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 7, 779–786 (2012). - PubMed
-
- Elsaesser A. & Howard C. V. Toxicology of nanoparticles. Adv. Drug Del. Rev. 64, 129–137 (2012). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

