AAV-mediated gene delivery in a feline model of Sandhoff disease corrects lysosomal storage in the central nervous system
- PMID: 25873306
- PMCID: PMC4720176
- DOI: 10.1177/1759091415569908
AAV-mediated gene delivery in a feline model of Sandhoff disease corrects lysosomal storage in the central nervous system
Abstract
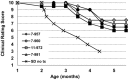
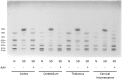
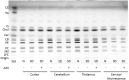
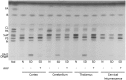
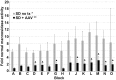
Sandhoff disease (SD) is an autosomal recessive neurodegenerative disease caused by a mutation in the gene for the β-subunit of β-N-acetylhexosaminidase (Hex), resulting in the inability to catabolize ganglioside GM2 within the lysosomes. SD presents with an accumulation of GM2 and its asialo derivative GA2, primarily in the central nervous system. Myelin-enriched glycolipids, cerebrosides and sulfatides, are also decreased in SD corresponding with dysmyelination. At present, no treatment exists for SD. Previous studies have shown the therapeutic benefit of adeno-associated virus (AAV) vector-mediated gene therapy in the treatment of SD in murine and feline models. In this study, we treated presymptomatic SD cats with AAVrh8 vectors expressing feline Hex in the thalamus combined with intracerebroventricular (Thal/ICV) injections. Treated animals showed clearly improved neurologic function and quality of life, manifested in part by prevention or attenuation of whole-body tremors characteristic of untreated animals. Hex activity was significantly elevated, whereas storage of GM2 and GA2 was significantly decreased in tissue samples taken from the cortex, cerebellum, thalamus, and cervical spinal cord. Treatment also increased levels of myelin-enriched cerebrosides and sulfatides in the cortex and thalamus. This study demonstrates the therapeutic potential of AAV for feline SD and suggests a similar potential for human SD patients.
Keywords: Sandhoff disease; adeno-associated virus; ganglioside; gene therapy; β-hexosaminidase.
© The Author(s) 2015.
Figures

References
-
- Ando S., Chang N. C., Yu R. K. (1978) High-performance thin-layer chromatography and densitometric determination of brain ganglioside compositions of several species. Analytical Biochemistry 89: 437–450. - PubMed
-
- Baek R. C., Kasperzyk J. L., Platt F. M., Seyfried T. N. (2008) N-butyldeoxygalactonojirimycin reduces brain ganglioside and GM2 content in neonatal Sandhoff disease mice. Neurochemistry International 52: 1125–1133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

