Comparison between the standard and a new alternative format of the Summary-of-Findings tables in Cochrane review users: study protocol for a randomized controlled trial
- PMID: 25873338
- PMCID: PMC4416250
- DOI: 10.1186/s13063-015-0649-6
Comparison between the standard and a new alternative format of the Summary-of-Findings tables in Cochrane review users: study protocol for a randomized controlled trial
Abstract
Background: Systematic reviews represent one of the most important tools for knowledge translation but users often struggle with understanding and interpreting their results. GRADE Summary-of-Findings tables have been developed to display results of systematic reviews in a concise and transparent manner. The current format of the Summary-of-Findings tables for presenting risks and quality of evidence improves understanding and assists users with finding key information from the systematic review. However, it has been suggested that additional methods to present risks and display results in the Summary-of-Findings tables are needed.
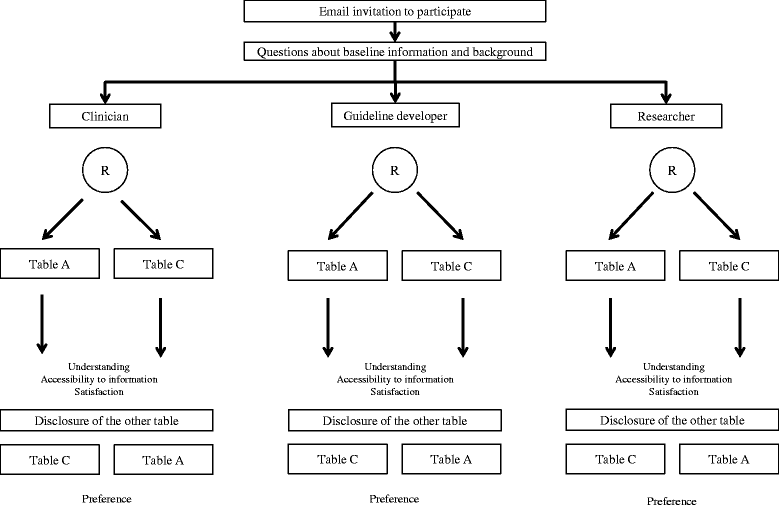
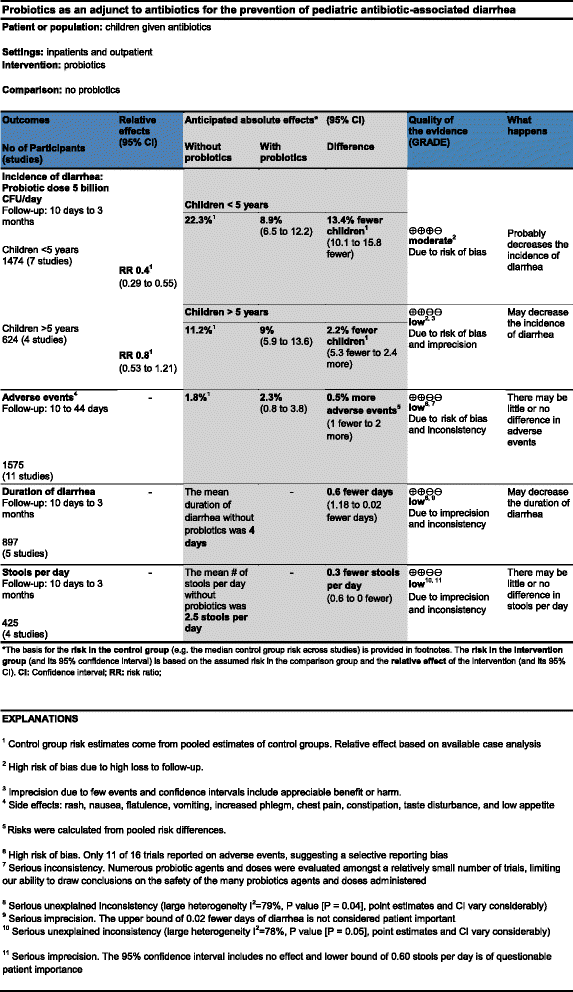
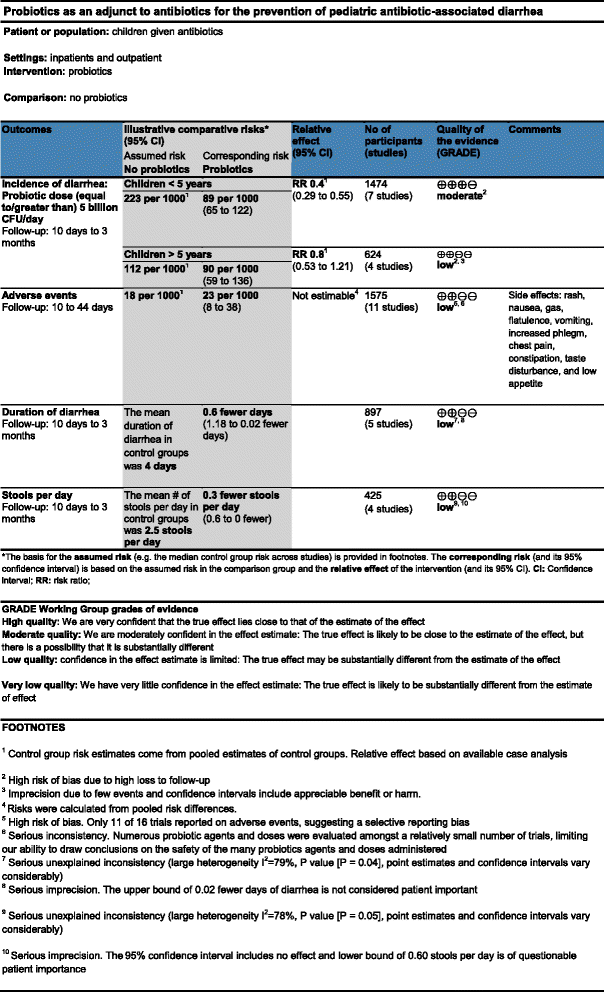
Methods/design: We will conduct a non-inferiority parallel-armed randomized controlled trial to determine whether an alternative format to present risks and display Summary-of-Findings tables is not inferior compared to the current standard format. We will measure participant understanding, accessibility of the information, satisfaction, and preference for both formats. We will invite systematic review users to participate (that is clinicians, guideline developers, and researchers). The data collection process will be undertaken using the online 'Survey Monkey' system. For the primary outcome understanding, non-inferiority of the alternative format (Table A) to the current standard format (Table C) of Summary-of-Findings tables will be claimed if the upper limit of a 1-sided 95% confidence interval (for the difference of proportion of participants answering correctly a given question) excluded a difference in favor of the current format of more than 10%.
Discussion: This study represents an effort to provide systematic reviewers with additional options to display review results using Summary-of-Findings tables. In this way, review authors will have a variety of methods to present risks and more flexibility to choose the most appropriate table features to display (that is optional columns, risks expressions, complementary methods to display continuous outcomes, and so on).
Trials registration: NCT02022631 (21 December 2013).
Figures
Similar articles
-
Improving GRADE evidence tables part 1: a randomized trial shows improved understanding of content in summary of findings tables with a new format.J Clin Epidemiol. 2016 Jun;74:7-18. doi: 10.1016/j.jclinepi.2015.12.007. Epub 2016 Jan 11. J Clin Epidemiol. 2016. PMID: 26791430 Clinical Trial.
-
Two alternatives versus the standard Grading of Recommendations Assessment, Development and Evaluation (GRADE) summary of findings (SoF) tables to improve understanding in the presentation of systematic review results: a three-arm, randomised, controlled, non-inferiority trial.BMJ Open. 2018 Jan 23;8(1):e015623. doi: 10.1136/bmjopen-2016-015623. BMJ Open. 2018. PMID: 29362242 Free PMC article. Clinical Trial.
-
Improving grading of recommendations assessment, development, and evaluation evidence tables part 4: a three-arm noninferiority randomized trial demonstrates improved understanding of content in summary of findings tables with a new format.J Clin Epidemiol. 2023 Feb;154:125-135. doi: 10.1016/j.jclinepi.2022.12.001. Epub 2022 Dec 8. J Clin Epidemiol. 2023. PMID: 36503004 Clinical Trial.
-
GRADE guidelines: 12. Preparing summary of findings tables-binary outcomes.J Clin Epidemiol. 2013 Feb;66(2):158-72. doi: 10.1016/j.jclinepi.2012.01.012. Epub 2012 May 18. J Clin Epidemiol. 2013. PMID: 22609141 Review.
-
GRADE pearls and pitfalls-Part 1: Systematic reviews and meta-analyses.Acta Anaesthesiol Scand. 2024 May;68(5):584-592. doi: 10.1111/aas.14386. Epub 2024 Feb 13. Acta Anaesthesiol Scand. 2024. PMID: 38351600 Review.
Cited by
-
Interactive Visual Displays for Interpreting the Results of Clinical Trials: Formative Evaluation With Case Vignettes.J Med Internet Res. 2018 Jun 25;20(6):e10507. doi: 10.2196/10507. J Med Internet Res. 2018. PMID: 29941416 Free PMC article.
-
Deworming drugs for soil-transmitted intestinal worms in children: effects on nutritional indicators, haemoglobin, and school performance.Cochrane Database Syst Rev. 2015 Jul 23;2015(7):CD000371. doi: 10.1002/14651858.CD000371.pub6. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2019 Sep 11;9:CD000371. doi: 10.1002/14651858.CD000371.pub7. PMID: 26202783 Free PMC article. Updated.
-
Knowledge mobilization activities to support decision-making by youth, parents, and adults using a systematic and living map of evidence and recommendations on COVID-19: protocol for three randomized controlled trials and qualitative user-experience studies.Trials. 2023 Jan 14;24(1):27. doi: 10.1186/s13063-023-07067-9. Trials. 2023. PMID: 36641457 Free PMC article.
-
School-based interventions for preventing HIV, sexually transmitted infections, and pregnancy in adolescents.Cochrane Database Syst Rev. 2016 Nov 8;11(11):CD006417. doi: 10.1002/14651858.CD006417.pub3. Cochrane Database Syst Rev. 2016. PMID: 27824221 Free PMC article.
-
High quality (certainty) evidence changes less often than low-quality evidence, but the magnitude of effect size does not systematically differ between studies with low versus high-quality evidence.J Eval Clin Pract. 2022 Jun;28(3):353-362. doi: 10.1111/jep.13657. Epub 2022 Jan 28. J Eval Clin Pract. 2022. PMID: 35089627 Free PMC article.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical