Cyclic AMP Response Element-binding Protein H (CREBH) Mediates the Inhibitory Actions of Tumor Necrosis Factor α in Osteoblast Differentiation by Stimulating Smad1 Degradation
- PMID: 25873397
- PMCID: PMC4505601
- DOI: 10.1074/jbc.M114.587923
Cyclic AMP Response Element-binding Protein H (CREBH) Mediates the Inhibitory Actions of Tumor Necrosis Factor α in Osteoblast Differentiation by Stimulating Smad1 Degradation
Abstract
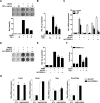
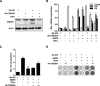
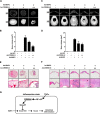
Endoplasmic reticulum (ER) stress transducers, such as old astrocyte specifically induced substance (OASIS) and activating transcription factor 6 (ATF6), which are induced by bone morphogenetic protein 2 (BMP2), regulate bone formation and osteoblast differentiation. Here, we examined the role of cAMP response element-binding protein H (CREBH), a member of the same family of ER membrane-bound basic leucine zipper (bZIP) transcription factors as OASIS and ATF6, in osteoblast differentiation and bone formation. Proinflammatory cytokine TNFα increased CREBH expression by up-regulating the nuclear factor-κB (NF-κB) signaling pathway in osteoblasts, increased the level of N-terminal fragment of CREBH in the nucleus, and inhibited BMP2 induction of osteoblast specific gene expression. Overexpression of CREBH suppressed BMP2-induced up-regulation of the osteogenic markers runt-related transcription factor 2 (Runx2), alkaline phosphatase (ALP), and osteocalcin (OC) in MC3T3-E1 cells and primary osteoblasts, as well as BMP2-induced ALP activity and OC protein production. In contrast, knockdown of CREBH attenuated the inhibitory effect of TNFα on BMP2-induced osteoblast differentiation. Mechanistic studies revealed that CREBH increased the expression of Smad ubiquitination regulatory factor 1 (Smurf1), leading to ubiquitin-dependent degradation of Smad1, whereas knockdown of CREBH inhibited TNFα-mediated degradation of Smad1 by Smurf1. Consistent with these in vitro findings, administration of Ad-CREBH inhibited BMP2-induced ectopic and orthotopic bone formation in vivo. Taken together, these results suggest that CREBH is a novel negative regulator of osteoblast differentiation and bone formation.
Keywords: SMAD transcription factor; cAMP response element-binding protein (CREB); endoplasmic reticulum stress (ER stress); osteoblast; tumor necrosis factor (TNF).
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



Similar articles
-
Tunicamycin negatively regulates BMP2-induced osteoblast differentiation through CREBH expression in MC3T3E1 cells.BMB Rep. 2011 Nov;44(11):735-40. doi: 10.5483/BMBRep.2011.44.11.735. BMB Rep. 2011. PMID: 22118540
-
Curcumin induces osteoblast differentiation through mild-endoplasmic reticulum stress-mediated such as BMP2 on osteoblast cells.Life Sci. 2018 Jan 15;193:34-39. doi: 10.1016/j.lfs.2017.12.008. Epub 2017 Dec 6. Life Sci. 2018. PMID: 29223538
-
CRTC2 suppresses BMP2-induced osteoblastic differentiation via Smurf1 expression in MC3T3-E1 cells.Life Sci. 2018 Dec 1;214:70-76. doi: 10.1016/j.lfs.2018.10.052. Epub 2018 Oct 26. Life Sci. 2018. PMID: 30449452
-
Physiological functions of endoplasmic reticulum stress transducer OASIS in central nervous system.Anat Sci Int. 2014 Jan;89(1):11-20. doi: 10.1007/s12565-013-0214-x. Epub 2013 Nov 16. Anat Sci Int. 2014. PMID: 24242870 Free PMC article. Review.
-
CREBH: A Complex Array of Regulatory Mechanisms in Nutritional Signaling, Metabolic Inflammation, and Metabolic Disease.Mol Nutr Food Res. 2021 Jan;65(1):e2000771. doi: 10.1002/mnfr.202000771. Epub 2020 Oct 20. Mol Nutr Food Res. 2021. PMID: 32997872 Review.
Cited by
-
Peroxiredoxin II negatively regulates BMP2-induced osteoblast differentiation and bone formation via PP2A Cα-mediated Smad1/5/9 dephosphorylation.Exp Mol Med. 2019 Jun 3;51(6):1-11. doi: 10.1038/s12276-019-0263-x. Exp Mol Med. 2019. PMID: 31160554 Free PMC article.
-
Extracellular S100A4 negatively regulates osteoblast function by activating the NF-κB pathway.BMB Rep. 2017 Feb;50(2):97-102. doi: 10.5483/bmbrep.2017.50.2.170. BMB Rep. 2017. PMID: 27998393 Free PMC article.
-
Cleavage of cyclic AMP-responsive element-binding protein H aggravates myocardial hypoxia reperfusion injury in a hepatocyte-myocardial cell co-culture system.J Int Med Res. 2020 May;48(5):300060520904835. doi: 10.1177/0300060520904835. J Int Med Res. 2020. Retraction in: J Int Med Res. 2024 Jan;52(1):3000605241228165. doi: 10.1177/03000605241228165. PMID: 32389049 Free PMC article. Retracted.
-
The Role of Mammalian Creb3-Like Transcription Factors in Response to Nutrients.Front Genet. 2019 Jun 21;10:591. doi: 10.3389/fgene.2019.00591. eCollection 2019. Front Genet. 2019. PMID: 31293620 Free PMC article. Review.
-
TGFβ1 Suppressed Matrix Mineralization of Osteoblasts Differentiation by Regulating SMURF1-C/EBPβ-DKK1 Axis.Int J Mol Sci. 2020 Dec 21;21(24):9771. doi: 10.3390/ijms21249771. Int J Mol Sci. 2020. PMID: 33371439 Free PMC article.
References
-
- Urist M. R. (1965) Bone: formation by autoinduction. Science 150, 893–899 - PubMed
-
- Wozney J. M., Rosen V., Celeste A. J., Mitsock L. M., Whitters M. J., Kriz R. W., Hewick R. M., Wang E. A. (1988) Novel regulators of bone formation: molecular clones and activities. Science 242, 1528–1534 - PubMed
-
- Ron D., Walter P. (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8, 519–529 - PubMed
-
- Harding H. P., Zhang Y., Ron D. (1999) Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397, 271–274 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

