Pepper aldehyde dehydrogenase CaALDH1 interacts with Xanthomonas effector AvrBsT and promotes effector-triggered cell death and defence responses
- PMID: 25873668
- PMCID: PMC4449550
- DOI: 10.1093/jxb/erv147
Pepper aldehyde dehydrogenase CaALDH1 interacts with Xanthomonas effector AvrBsT and promotes effector-triggered cell death and defence responses
Abstract
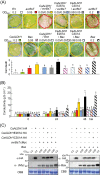
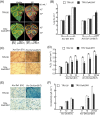
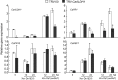
Xanthomonas type III effector AvrBsT induces hypersensitive cell death and defence responses in pepper (Capsicum annuum) and Nicotiana benthamiana. Little is known about the host factors that interact with AvrBsT. Here, we identified pepper aldehyde dehydrogenase 1 (CaALDH1) as an AvrBsT-interacting protein. Bimolecular fluorescence complementation and co-immunoprecipitation assays confirmed the interaction between CaALDH1 and AvrBsT in planta. CaALDH1:smGFP fluorescence was detected in the cytoplasm. CaALDH1 expression in pepper was rapidly and strongly induced by avirulent Xanthomonas campestris pv. vesicatoria (Xcv) Ds1 (avrBsT) infection. Transient co-expression of CaALDH1 with avrBsT significantly enhanced avrBsT-triggered cell death in N. benthamiana leaves. Aldehyde dehydrogenase activity was higher in leaves transiently expressing CaALDH1, suggesting that CaALDH1 acts as a cell death enhancer, independently of AvrBsT. CaALDH1 silencing disrupted phenolic compound accumulation, H2O2 production, defence response gene expression, and cell death during avirulent Xcv Ds1 (avrBsT) infection. Transgenic Arabidopsis thaliana overexpressing CaALDH1 exhibited enhanced defence response to Pseudomonas syringae pv. tomato and Hyaloperonospora arabidopsidis infection. These results indicate that cytoplasmic CaALDH1 interacts with AvrBsT and promotes plant cell death and defence responses.
Keywords: Aldehyde dehydrogenase; Xanthomonas campestris pv. vesicatoria.; cell death; effector AvrBsT; pepper; plant defence.
© The Author 2015. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures








References
-
- Chen TH, Murata N. 2002. Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Current Opinion in Plant Biology 5, 250–257. - PubMed
-
- Chen XB, Zeng Q, Wood AJ. 2002. The stress-responsive Tortula ruralis gene ALDH21A1 describes a novel eukaryotic aldehyde dehydrogenase protein family. Journal of Plant Physiology 159, 677–684.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

