Sucrose is an early modulator of the key hormonal mechanisms controlling bud outgrowth in Rosa hybrida
- PMID: 25873679
- PMCID: PMC4986866
- DOI: 10.1093/jxb/erv047
Sucrose is an early modulator of the key hormonal mechanisms controlling bud outgrowth in Rosa hybrida
Abstract
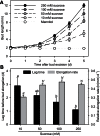
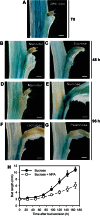
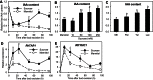
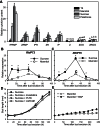
Sugar has only recently been identified as a key player in triggering bud outgrowth, while hormonal control of bud outgrowth is already well established. To get a better understanding of sugar control, the present study investigated how sugar availability modulates the hormonal network during bud outgrowth in Rosa hybrida. Other plant models, for which mutants are available, were used when necessary. Buds were grown in vitro to manipulate available sugars. The temporal patterns of the hormonal regulatory network were assessed in parallel with bud outgrowth dynamics. Sucrose determined bud entrance into sustained growth in a concentration-dependent manner. Sustained growth was accompanied by sustained auxin production in buds, and sustained auxin export in a DR5::GUS-expressing pea line. Several events occurred ahead of sucrose-stimulated bud outgrowth. Sucrose upregulated early auxin synthesis genes (RhTAR1, RhYUC1) and the auxin efflux carrier gene RhPIN1, and promoted PIN1 abundance at the plasma membrane in a pPIN1::PIN1-GFP-expressing tomato line. Sucrose downregulated both RwMAX2, involved in the strigolactone-transduction pathway, and RhBRC1, a repressor of branching, at an early stage. The presence of sucrose also increased stem cytokinin content, but sucrose-promoted bud outgrowth was not related to that pathway. In these processes, several non-metabolizable sucrose analogues induced sustained bud outgrowth in R. hybrida, Pisum sativum, and Arabidopsis thaliana, suggesting that sucrose was involved in a signalling pathway. In conclusion, we identified potential hormonal candidates for bud outgrowth control by sugar. They are central to future investigations aimed at disentangling the processes that underlie regulation of bud outgrowth by sugar.
Keywords: Auxin; Rosa sp.; bud burst; cytokinins; shoot branching; strigolactones; sugar; sugar signalling..
© The Author 2015. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures



References
-
- Arrom L, Munné-Bosch S. 2012a. Sucrose accelerates flower opening and delays senescence through a hormonal effect in cut lily flowers. Plant Science 188–189, 41–47. - PubMed
-
- Arrom L, Munné-Bosch S. 2012b. Hormonal changes during flower development in floral tissues of Lilium. Planta 236, 343–354. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

