Toll-like receptor-4 mediated inflammation is involved in the cardiometabolic alterations induced by intermittent hypoxia
- PMID: 25873766
- PMCID: PMC4383499
- DOI: 10.1155/2015/620258
Toll-like receptor-4 mediated inflammation is involved in the cardiometabolic alterations induced by intermittent hypoxia
Abstract
Objective: Intermittent hypoxia (IH) is a major component of sleep apnea syndrome as its cardiometabolic complications have been mainly attributed to IH. The pathophysiology is still poorly understood but there are some similarities with the obesity-associated cardiometabolic complications. As the latter results from inflammation involving toll-like receptor-4 (TLR4) signaling, we assessed this pathway in the cardiometabolic consequences of IH.
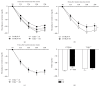
Methods: Lean adult male TLR4-deficient (TLR4(-/-)) mice and their controls (C57BL/6 mice) were exposed to either IH (FiO2 21-5%, 1 min cycle, 8 h/day) or air (normoxic mice) for 4 weeks. Animals were assessed at 1-week exposure for insulin tolerance test and after 4-week exposure for morphological and inflammatory changes of the epididymal fat and thoracic aorta.
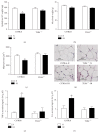
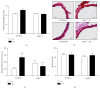
Results: IH induced insulin resistance, morphological and inflammatory changes of the epididymal fat (smaller pads and adipocytes, higher release of TNF-α and IL-6) and aorta (larger intima-media thickness and higher NFκB-p50 activity). All these alterations were prevented by TLR4 deletion.
Conclusion: IH induces metabolic and vascular alterations that involve TLR4 mediated inflammation. These results confirm the important role of inflammation in the cardiometabolic consequences of IH and suggest that targeting TLR4/NFκB pathway could represent a further therapeutic option for sleep apnea patients.
Figures
References
-
- Somers V. K., White D. P., Amin R., et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health) Circulation. 2008;118(10):1080–1111. doi: 10.1161/CIRCULATIONAHA.107.189420. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

