Changes in the regulation of the Notch signaling pathway are temporally correlated with regenerative failure in the mouse cochlea
- PMID: 25873862
- PMCID: PMC4379755
- DOI: 10.3389/fncel.2015.00110
Changes in the regulation of the Notch signaling pathway are temporally correlated with regenerative failure in the mouse cochlea
Abstract
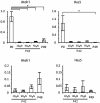
Sensorineural hearing loss is most commonly caused by the death of hair cells in the organ of Corti, and once lost, mammalian hair cells do not regenerate. In contrast, other vertebrates such as birds can regenerate hair cells by stimulating division and differentiation of neighboring supporting cells. We currently know little of the genetic networks which become active in supporting cells when hair cells die and that are activated in experimental models of hair cell regeneration. Several studies have shown that neonatal mammalian cochlear supporting cells are able to trans-differentiate into hair cells when cultured in conditions in which the Notch signaling pathway is blocked. We now show that the ability of cochlear supporting cells to trans-differentiate declines precipitously after birth, such that supporting cells from six-day-old mouse cochlea are entirely unresponsive to a blockade of the Notch pathway. We show that this trend is seen regardless of whether the Notch pathway is blocked with gamma secretase inhibitors, or by antibodies against the Notch1 receptor, suggesting that the action of gamma secretase inhibitors on neonatal supporting cells is likely to be by inhibiting Notch receptor cleavage. The loss of responsiveness to inhibition of the Notch pathway in the first postnatal week is due in part to a down-regulation of Notch receptors and ligands, and we show that this down-regulation persists in the adult animal, even under conditions of noise damage. Our data suggest that the Notch pathway is used to establish the repeating pattern of hair cells and supporting cells in the organ of Corti, but is not required to maintain this cellular mosaic once the production of hair cells and supporting cells is completed. Our results have implications for the proposed used of Notch pathway inhibitors in hearing restoration therapies.
Keywords: cochlea; hair cell; notch; regeneration; supporting cell.
Figures

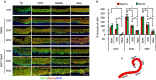
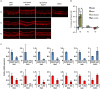

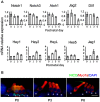
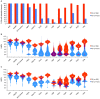
References
-
- Adam J., Myat A., Le Roux I., Eddison M., Henrique D., Ish-Horowicz D., et al. . (1998). Cell fate choices and the expression of Notch, Delta and Serrate homologues in the chick inner ear: parallels with Drosophila sense-organ development. Development 125, 4645–4654. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

