Large-scale production and protein engineering of G protein-coupled receptors for structural studies
- PMID: 25873898
- PMCID: PMC4379943
- DOI: 10.3389/fphar.2015.00066
Large-scale production and protein engineering of G protein-coupled receptors for structural studies
Abstract
Structural studies of G protein-coupled receptors (GPCRs) gave insights into molecular mechanisms of their action and contributed significantly to molecular pharmacology. This is primarily due to technical advances in protein engineering, production and crystallization of these important receptor targets. On the other hand, NMR spectroscopy of GPCRs, which can provide information about their dynamics, still remains challenging due to difficulties in preparation of isotopically labeled receptors and their low long-term stabilities. In this review, we discuss methods used for expression and purification of GPCRs for crystallographic and NMR studies. We also summarize protein engineering methods that played a crucial role in obtaining GPCR crystal structures.
Keywords: G protein-coupled receptor (GPCR); expression; isotope labeling; protein engineering; purification.
Figures
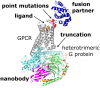
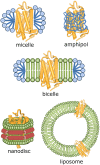

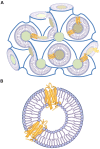

References
-
- André N., Cherouati N., Prual C., Steffan T., Zeder-Lutz G., Magnin T., et al. (2006). Enhancing functional production of G protein-coupled receptors in Pichia pastoris to levels required for structural studies via a single expression screen. Protein Sci. 15 1115–1126 10.1110/ps.062098206 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

