Antifungal and antibacterial metabolites from a French poplar type propolis
- PMID: 25873978
- PMCID: PMC4385655
- DOI: 10.1155/2015/319240
Antifungal and antibacterial metabolites from a French poplar type propolis
Abstract
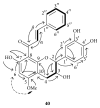
During this study, the in vitro antifungal and antibacterial activities of different extracts (aqueous and organic) obtained from a French propolis batch were evaluated. Antifungal activity was evaluated by broth microdilution on three pathogenic strains: Candida albicans, C. glabrata, and Aspergillus fumigatus. Antibacterial activity was assayed using agar dilution method on 36 Gram-negative and Gram-positive strains including Staphylococcus aureus. Organic extracts showed a significant antifungal activity against C. albicans and C. glabrata (MIC80 between 16 and 31 µg/mL) but only a weak activity towards A. fumigatus (MIC80 = 250 µg/mL). DCM based extracts exhibited a selective Gram-positive antibacterial activity, especially against S. aureus (SA) and several of its methicillin-resistant (MRSA) and methicillin-susceptible (MSSA) strains (MIC100 30-97 µg/mL). A new and active derivative of catechin was also identified whereas a synergistic antimicrobial effect was noticed during this study.
Figures



References
-
- Cottica S. M., Sawaya A. C. H. F., Eberlin M. N., Franco S. L., Zeoula L. M., Visentainer J. V. Antioxidant activity and composition of propolis obtained by different methods of extraction. Journal of the Brazilian Chemical Society. 2011;22(5):929–935. doi: 10.1590/S0103-50532011000500016. - DOI
-
- Sawaya A. C. H. F., Palma A. M., Caetano F. M., et al. Comparative study of in vitro methods used to analyse the activity of propolis extracts with different compositions against species of Candida. Letters in Applied Microbiology. 2002;35(3):203–207. doi: 10.1046/j.1472-765X.2002.01169.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

