A Potent Systemically Active N-Acylethanolamine Acid Amidase Inhibitor that Suppresses Inflammation and Human Macrophage Activation
- PMID: 25874594
- PMCID: PMC4546552
- DOI: 10.1021/acschembio.5b00114
A Potent Systemically Active N-Acylethanolamine Acid Amidase Inhibitor that Suppresses Inflammation and Human Macrophage Activation
Abstract
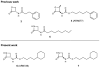
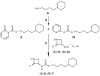
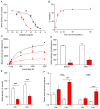
Fatty acid ethanolamides such as palmitoylethanolamide (PEA) and oleoylethanolamide (OEA) are lipid-derived mediators that potently inhibit pain and inflammation by ligating type-α peroxisome proliferator-activated receptors (PPAR-α). These bioactive substances are preferentially degraded by the cysteine hydrolase, N-acylethanolamine acid amidase (NAAA), which is highly expressed in macrophages. Here, we describe a new class of β-lactam derivatives that are potent, selective, and systemically active inhibitors of intracellular NAAA activity. The prototype of this class deactivates NAAA by covalently binding the enzyme's catalytic cysteine and exerts profound anti-inflammatory effects in both mouse models and human macrophages. This agent may be used to probe the functions of NAAA in health and disease and as a starting point to discover better anti-inflammatory drugs.
Figures
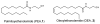

References
-
- Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, Rodríguez De Fonseca F, Rosengarth A, Luecke H, Di Giacomo B, Tarzia G, Piomelli D. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature. 2003;425:90–93. - PubMed
-
- Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, Piomelli D. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol. 2005;67:15–19. - PubMed
-
- D'Agostino G, La Rana G, Russo R, Sasso O, Iacono A, Esposito E, Raso GM, Cuzzocrea S, Lo Verme J, Piomelli D, Meli R, Calignano A. Acute intracerebroventricular administration of palmitoylethanolamide, an endogenous peroxisome proliferator-activated receptor-alpha agonist, modulates carrageenan-induced paw edema in mice. J Pharmacol Exp Ther. 2007;322:1137–1143. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

