Oxytocin enables maternal behaviour by balancing cortical inhibition
- PMID: 25874674
- PMCID: PMC4409554
- DOI: 10.1038/nature14402
Oxytocin enables maternal behaviour by balancing cortical inhibition
Abstract
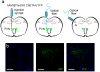
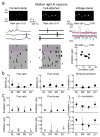
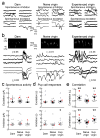
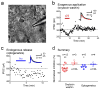
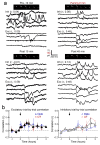
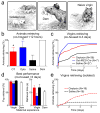
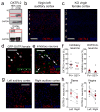
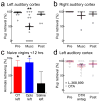
Oxytocin is important for social interactions and maternal behaviour. However, little is known about when, where and how oxytocin modulates neural circuits to improve social cognition. Here we show how oxytocin enables pup retrieval behaviour in female mice by enhancing auditory cortical pup call responses. Retrieval behaviour required the left but not right auditory cortex, was accelerated by oxytocin in the left auditory cortex, and oxytocin receptors were preferentially expressed in the left auditory cortex. Neural responses to pup calls were lateralized, with co-tuned and temporally precise excitatory and inhibitory responses in the left cortex of maternal but not pup-naive adults. Finally, pairing calls with oxytocin enhanced responses by balancing the magnitude and timing of inhibition with excitation. Our results describe fundamental synaptic mechanisms by which oxytocin increases the salience of acoustic social stimuli. Furthermore, oxytocin-induced plasticity provides a biological basis for lateralization of auditory cortical processing.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




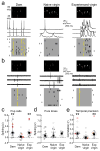
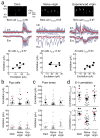
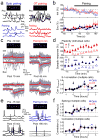
Comment in
-
Sensory systems: The yin and yang of cortical oxytocin.Nature. 2015 Apr 23;520(7548):444-5. doi: 10.1038/nature14386. Epub 2015 Apr 15. Nature. 2015. PMID: 25874677 Free PMC article.
-
Behavioural neuroscience: Picking up the pups.Nat Rev Neurosci. 2015 Jun;16(6):315. doi: 10.1038/nrn3967. Epub 2015 May 8. Nat Rev Neurosci. 2015. PMID: 25953681 No abstract available.
References
-
- Richard P, Moos F, Freund-Mercier MJ. Central effects of oxytocin. Physiol Rev. 1991;71:331–370. - PubMed
-
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. - PubMed
-
- Insel TR, Young LJ. The neurobiology of attachment. Nat Rev Neurosci. 2001;2:129–136. - PubMed
-
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: Context and person matter. Trends Cogn Sci. 2011;15:301–309. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

