The role of lipoprotein-associated phospholipase A2 in a murine model of experimental autoimmune uveoretinitis
- PMID: 25874928
- PMCID: PMC4398387
- DOI: 10.1371/journal.pone.0122093
The role of lipoprotein-associated phospholipase A2 in a murine model of experimental autoimmune uveoretinitis
Abstract
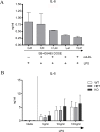
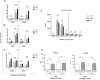
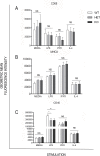
Macrophage activation is, in part, regulated via hydrolysis of oxidised low density lipoproteins by Lipoprotein-Associated phospholipase A2 (Lp-PLA2), resulting in increased macrophage migration, pro-inflammatory cytokine release and chemokine expression. In uveitis, tissue damage is mediated as a result of macrophage activation; hence inhibition of Lp-PLA2 may limit macrophage activation and protect the tissue. Utilising Lp-PLA2 gene-deficient (KO) mice and a pharmacological inhibitor of Lp-PLA2 (SB-435495) we aimed to determine the effect of Lp-PLA2 suppression in mediating retinal protection in a model of autoimmune retinal inflammation, experimental autoimmune uveoretinitis (EAU). Following immunisation with RBP-3 (IRBP) 1-20 or 161-180 peptides, clinical disease was monitored and severity assessed, infiltrating leukocytes were enumerated by flow cytometry and tissue destruction quantified by histology. Despite ablation of Lp-PLA2 enzyme activity in Lp-PLA2 KO mice or wild-type mice treated with SB-435495, the number of infiltrating CD45+ cells in the retina was equivalent to control EAU animals, and there was no reduction in disease severity. Thus, despite the reported beneficial effects of therapeutic Lp-PLA2 depletion in a variety of vascular inflammatory conditions, we were unable to attenuate disease, show delayed disease onset or prevent progression of EAU in Lp-PLA2 KO mice. Although EAU exhibits inflammatory vasculopathy there is no overt defect in lipid metabolism and given the lack of effect following Lp-PLA2 suppression, these data support the hypothesis that sub-acute autoimmune inflammatory disease progresses independently of Lp-PLA2 activity.
Conflict of interest statement
Figures




Similar articles
-
Lipoprotein-associated phospholipase A2 (Lp-PLA2) as a therapeutic target to prevent retinal vasopermeability during diabetes.Proc Natl Acad Sci U S A. 2016 Jun 28;113(26):7213-8. doi: 10.1073/pnas.1514213113. Epub 2016 Jun 13. Proc Natl Acad Sci U S A. 2016. PMID: 27298369 Free PMC article.
-
Experimental autoimmune uveoretinitis (EAU)-related tissue damage and angiogenesis is reduced in CCL2⁻/⁻CX₃CR1gfp/gfp mice.Invest Ophthalmol Vis Sci. 2014 Oct 23;55(11):7572-82. doi: 10.1167/iovs.14-15495. Invest Ophthalmol Vis Sci. 2014. PMID: 25342612
-
Monoclonal antibody-mediated CD200 receptor signaling suppresses macrophage activation and tissue damage in experimental autoimmune uveoretinitis.Am J Pathol. 2007 Aug;171(2):580-8. doi: 10.2353/ajpath.2007.070272. Epub 2007 Jun 28. Am J Pathol. 2007. PMID: 17600119 Free PMC article.
-
[Lipoprotein-associated phospholipase A2 (Lp-PLA2): Relevant biomarker and therapeutic target?].Ann Pharm Fr. 2025 Jan;83(1):45-57. doi: 10.1016/j.pharma.2024.08.011. Epub 2024 Sep 4. Ann Pharm Fr. 2025. PMID: 39241907 Review. French.
-
Lipoprotein-associated phospholipase A(2) and atherosclerosis.Curr Opin Lipidol. 2009 Oct;20(5):415-20. doi: 10.1097/MOL.0b013e3283307c16. Curr Opin Lipidol. 2009. PMID: 19667981 Review.
Cited by
-
Lipoprotein-associated phospholipase A2 (Lp-PLA2) as a therapeutic target to prevent retinal vasopermeability during diabetes.Proc Natl Acad Sci U S A. 2016 Jun 28;113(26):7213-8. doi: 10.1073/pnas.1514213113. Epub 2016 Jun 13. Proc Natl Acad Sci U S A. 2016. PMID: 27298369 Free PMC article.
-
Lipoprotein-associated phospholipase A2: The story continues.Med Res Rev. 2020 Jan;40(1):79-134. doi: 10.1002/med.21597. Epub 2019 May 29. Med Res Rev. 2020. PMID: 31140638 Free PMC article. Review.
-
Combination of theoretical analysis and experiments: Exploring the role of PLA2G7 in human cancers, including renal cancer.Heliyon. 2024 Mar 9;10(6):e27906. doi: 10.1016/j.heliyon.2024.e27906. eCollection 2024 Mar 30. Heliyon. 2024. PMID: 38509948 Free PMC article.
-
Ocular diseases: immunological and molecular mechanisms.Int J Ophthalmol. 2016 May 18;9(5):780-8. doi: 10.18240/ijo.2016.05.25. eCollection 2016. Int J Ophthalmol. 2016. PMID: 27275439 Free PMC article. Review.
References
-
- (WHO) WHO. Global data on visual impairments 2010 [cited 2014 July 01].
-
- Yeo TK, Ho SL, Lim WK, Teoh SC. Causes of Visual Loss Associated with Uveitis in a Singapore Tertiary Eye Center. Ocular Immunology & Inflammation. 2013;21(4):264–9. - PubMed
-
- Shewitz-Bowers L, Lee R.W.J., Dick A.D. Immune mechanisms of intraocular inflammation. Expert Reviews. 2010;5(1):43–58.
-
- Forrester JV, Liversidge J, Dick A, McMenamin P, Kuppner M, Crane I, et al. What determines the site of inflammation in uveitis and chorioretinitis? Eye. 1997;11(2):162–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

