Cajal body proteins differentially affect the processing of box C/D scaRNPs
- PMID: 25875178
- PMCID: PMC4395269
- DOI: 10.1371/journal.pone.0122348
Cajal body proteins differentially affect the processing of box C/D scaRNPs
Abstract
Small nuclear ribonucleoproteins (snRNPs), which are required for pre-mRNA splicing, contain extensively modified snRNA. Small Cajal body-specific ribonucleoproteins (scaRNPs) mediate these modifications. It is unknown how the box C/D class of scaRNPs localizes to Cajal Bodies (CBs). The processing of box C/D scaRNA is also unclear. Here, we explore the processing of box C/D scaRNA 2 and 9 by coilin. We also broaden our investigation to include WRAP53 and SMN, which accumulate in CBs, play a role in RNP biogenesis and associate with coilin. These studies demonstrate that the processing of an ectopically expressed scaRNA2 is altered upon the reduction of coilin, WRAP53 or SMN, but the extent and direction of this change varies depending on the protein reduced. We also show that box C/D scaRNP activity is reduced in a cell line derived from coilin knockout mice. Collectively, the findings presented here further implicate coilin as being a direct participant in the formation of box C/D scaRNPs, and demonstrate that WRAP53 and SMN may also play a role, but the activity of these proteins is divergent to coilin.
Conflict of interest statement
Figures
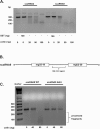


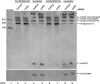
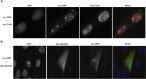


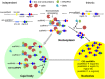
Similar articles
-
Identification of processing elements and interactors implicate SMN, coilin and the pseudogene-encoded coilp1 in telomerase and box C/D scaRNP biogenesis.RNA Biol. 2016 Oct 2;13(10):955-972. doi: 10.1080/15476286.2016.1211224. Epub 2016 Jul 15. RNA Biol. 2016. PMID: 27419845 Free PMC article.
-
Residual Cajal bodies in coilin knockout mice fail to recruit Sm snRNPs and SMN, the spinal muscular atrophy gene product.J Cell Biol. 2001 Jul 23;154(2):293-307. doi: 10.1083/jcb.200104083. J Cell Biol. 2001. PMID: 11470819 Free PMC article.
-
Ongoing U snRNP biogenesis is required for the integrity of Cajal bodies.Mol Biol Cell. 2006 Jul;17(7):3221-31. doi: 10.1091/mbc.e06-03-0247. Epub 2006 May 10. Mol Biol Cell. 2006. PMID: 16687569 Free PMC article.
-
Towards an understanding of regulating Cajal body activity by protein modification.RNA Biol. 2017 Jun 3;14(6):761-778. doi: 10.1080/15476286.2016.1243649. Epub 2016 Oct 7. RNA Biol. 2017. PMID: 27819531 Free PMC article. Review.
-
Pumping RNA: nuclear bodybuilding along the RNP pipeline.Curr Opin Cell Biol. 2006 Jun;18(3):317-24. doi: 10.1016/j.ceb.2006.03.005. Epub 2006 May 2. Curr Opin Cell Biol. 2006. PMID: 16632338 Review.
Cited by
-
Biology and clinical relevance of noncoding sno/scaRNAs.Trends Cardiovasc Med. 2018 Feb;28(2):81-90. doi: 10.1016/j.tcm.2017.08.002. Epub 2017 Aug 12. Trends Cardiovasc Med. 2018. PMID: 28869095 Free PMC article. Review.
-
The Cajal Body Protein WRAP53β Prepares the Scene for Repair of DNA Double-Strand Breaks by Regulating Local Ubiquitination.Front Mol Biosci. 2019 Jul 4;6:51. doi: 10.3389/fmolb.2019.00051. eCollection 2019. Front Mol Biosci. 2019. PMID: 31334247 Free PMC article. Review.
-
Alteration of 28S rRNA 2'-O-methylation by etoposide correlates with decreased SMN phosphorylation and reduced Drosha levels.Biol Open. 2019 Mar 27;8(3):bio041848. doi: 10.1242/bio.041848. Biol Open. 2019. PMID: 30858166 Free PMC article.
-
Altered dynamics of scaRNA2 and scaRNA9 in response to stress correlates with disrupted nuclear organization.Biol Open. 2018 Sep 27;7(9):bio037101. doi: 10.1242/bio.037101. Biol Open. 2018. PMID: 30177550 Free PMC article.
-
Identification of processing elements and interactors implicate SMN, coilin and the pseudogene-encoded coilp1 in telomerase and box C/D scaRNP biogenesis.RNA Biol. 2016 Oct 2;13(10):955-972. doi: 10.1080/15476286.2016.1211224. Epub 2016 Jul 15. RNA Biol. 2016. PMID: 27419845 Free PMC article.
References
-
- Liang XH, Liu Q, Fournier MJ. rRNA modifications in an intersubunit bridge of the ribosome strongly affect both ribosome biogenesis and activity. Mol Cell. 2007;28: 965–977. - PubMed
-
- Kiss T. Biogenesis of small nuclear RNPs. J Cell Sci. 2004;117: 5949–5951. - PubMed
-
- Fischer U, Liu Q, Dreyfuss G. The SMN-SIP1 complex has an essential role in spliceosomal snRNP biogenesis. Cell. 1997;90: 1023–1029. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

