A tetravalent bispecific TandAb (CD19/CD3), AFM11, efficiently recruits T cells for the potent lysis of CD19(+) tumor cells
- PMID: 25875246
- PMCID: PMC4622993
- DOI: 10.1080/19420862.2015.1029216
A tetravalent bispecific TandAb (CD19/CD3), AFM11, efficiently recruits T cells for the potent lysis of CD19(+) tumor cells
Abstract
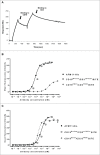
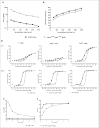
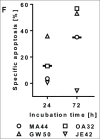
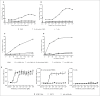
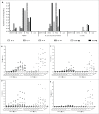
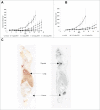
To harness the potent tumor-killing capacity of T cells for the treatment of CD19(+) malignancies, we constructed AFM11, a humanized tetravalent bispecific CD19/CD3 tandem diabody (TandAb) consisting solely of Fv domains. The molecule exhibits good manufacturability and stability properties. AFM11 has 2 binding sites for CD3 and 2 for CD19, an antigen that is expressed from early B cell development through differentiation into plasma cells, and is an attractive alternative to CD20 as a target for the development of therapeutic antibodies to treat B cell malignancies. Comparison of the binding and cytotoxicity of AFM11 with those of a tandem scFv bispecific T cell engager (BiTE) molecule targeting the same antigens revealed that AFM11 elicited more potent in vitro B cell lysis. Though possessing high affinity to CD3, the TandAb mediates serial-killing of CD19(+) cells with little dependence of potency or efficacy upon effector:target ratio, unlike the BiTE. The advantage of the TandAb over the BiTE was most pronounced at lower effector:target ratios. AFM11 mediated strictly target-dependent T cell activation evidenced by CD25 and CD69 induction, proliferation, and cytokine release, notwithstanding bivalent CD3 engagement. In a NOD/scid xenograft model, AFM11 induced dose-dependent growth inhibition of Raji tumors in vivo, and radiolabeled TandAb exhibited excellent localization to tumor but not to normal tissue. After intravenous administration in mice, half-life ranged from 18.4 to 22.9 h. In a human ex vivo B-cell chronic lymphocytic leukemia study, AFM11 exhibited substantial cytotoxic activity in an autologous setting. Thus, AFM11 may represent a promising therapeutic for treatment of CD19(+) malignancies with an advantageous safety risk profile and anticipated dosing regimen.
Keywords: ALL; AUCtot, total area under the curve; B-ALL, B-precursor acute lymphoblastic leukemia; BBB, blood-brain barrier; BiTE, bispecific T cell engager; CAR, chimeric antigen receptor; CCS, cell culture supernatant; CD, cluster of differentiation; CD3; CDR, complementarity determining region; CHO, Chinese hamster ovary; CL, clearance; CLL, chronic lymphocytic leukemia; CNS, central nervous system; Cmax, maximal concentration; DMSO, dimethyl sulfoxide; E:T, effector:target; EC50, half maximal effective concentration; ECL, electrochemiluminescence; F, fluorescence; FACS, fluorescence-activated cell sorting; FCS, fetal calf serum; FR, framework region; Fab, fragment antigen-binding; Fc, fragment crystallizable; FcRn, neonatal Fc receptor; FcgR, Fc gamma receptor; Fv, variable fragment; HMF, high molecular weight forms; HSA, human serum albumin; His, histidine; IFN, interferon; IL, interleukin; IgG, immunoglobulin G; KD, dissociation constant; LMF, low molecular weight forms; MSD, MesoScale Discovery; MWCO, molecular weight cut-off; NHL, non-Hodgkin lymphoma; NK, natural killer; NOD/scid, nonobese diabetic/severe combined immunodeficiency; Non-Hodgkin lymphoma; ORR, overall response rate; PBMC, peripheral blood mononuclear cell; PBS, phosphate buffered saline; PES, polyethersulfone; PHA, phytohemagglutinin; PI, propidium iodide; SABC, standardized antibody binding capacity; SD, standard deviation; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis; SE-HPLC, size exclusion high-pressure liquid chromatography; SEC, size exclusion chromatography; SPR, surface plasmon resonance; T cells; TNF, tumor necrosis factor; TandAb, tandem diabody; VH, variable heavy; VL, variable light; Vss, volume of distribution at steady state; WBA, whole body autoradiography; bispecific antibodies; ctrl., control; i.v., intravenous; ka, association rate constant; kd, dissociation rate constant; s.c., subcutaneous; scFv, single-chain variable fragment; t1/2, terminal elimination half-life; w/o, without.
Figures

References
-
- Grillo-López AJ, White CA, Dallaire BK, Varns CL, Shen CD, Wei A, Leonard JE, McClure A, Weaver R, Cairelli S, et al.. Rituximab: the first monoclonal antibody approved for the treatment of lymphoma. Curr Pharm Biotechnol 2000; 1:1-9; PMID:11467356; http://dx.doi.org/2120938010.2174/1389201003379059 - DOI - PubMed
-
- Alduaij W, Illidge TM. The future of anti-CD20 monoclonal antibodies: are we making progress?. Blood 2011; 117:2993-3001; PMID:21209380; http://dx.doi.org/10.1182/blood-2010-07-298356 - DOI - PubMed
-
- Illidge T, Cheadle EJ, Donaghy C, Honeychurch J. Update on obinutuzumab in the treatment of B-cell malignancies. Expert Opin Biol Ther 2014; 14:1507-17; PMID:25190612; http://dx.doi.org/10.1517/14712598.2014.948414 - DOI - PubMed
-
- Remer M, Al-Shamkhani A, Glennie M, Johnson P. Mogamulizumab and the treatment of ccr4-positive t-cell lymphomas. Immunotherapy 2014; 6:1187-206; PMID:25496334; http://dx.doi.org/10.2217/imt.14.94 - DOI - PubMed
-
- Horton HM, Bernett MJ, Pong E, Peipp M, Karki S, Chu SY, Richards JO, Vostiar I, Joyce PF, Repp R, et al.. Potent in vitro and in vivo activity of an Fc-engineered anti-CD19 monoclonal antibody against lymphoma and leukemia. Cancer Res 2008; 68:8049-57; PMID:18829563; http://dx.doi.org/10.1158/0008-5472.CAN-08-2268 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
