Spastin binds to lipid droplets and affects lipid metabolism
- PMID: 25875445
- PMCID: PMC4395272
- DOI: 10.1371/journal.pgen.1005149
Spastin binds to lipid droplets and affects lipid metabolism
Abstract
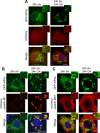
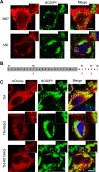
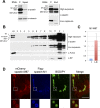
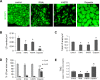
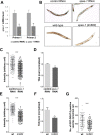
Mutations in SPAST, encoding spastin, are the most common cause of autosomal dominant hereditary spastic paraplegia (HSP). HSP is characterized by weakness and spasticity of the lower limbs, owing to progressive retrograde degeneration of the long corticospinal axons. Spastin is a conserved microtubule (MT)-severing protein, involved in processes requiring rearrangement of the cytoskeleton in concert to membrane remodeling, such as neurite branching, axonal growth, midbody abscission, and endosome tubulation. Two isoforms of spastin are synthesized from alternative initiation codons (M1 and M87). We now show that spastin-M1 can sort from the endoplasmic reticulum (ER) to pre- and mature lipid droplets (LDs). A hydrophobic motif comprised of amino acids 57 through 86 of spastin was sufficient to direct a reporter protein to LDs, while mutation of arginine 65 to glycine abolished LD targeting. Increased levels of spastin-M1 expression reduced the number but increased the size of LDs. Expression of a mutant unable to bind and sever MTs caused clustering of LDs. Consistent with these findings, ubiquitous overexpression of Dspastin in Drosophila led to bigger and less numerous LDs in the fat bodies and increased triacylglycerol levels. In contrast, Dspastin overexpression increased LD number when expressed specifically in skeletal muscles or nerves. Downregulation of Dspastin and expression of a dominant-negative variant decreased LD number in Drosophila nerves, skeletal muscle and fat bodies, and reduced triacylglycerol levels in the larvae. Moreover, we found reduced amount of fat stores in intestinal cells of worms in which the spas-1 homologue was either depleted by RNA interference or deleted. Taken together, our data uncovers an evolutionarily conserved role of spastin as a positive regulator of LD metabolism and open up the possibility that dysfunction of LDs in axons may contribute to the pathogenesis of HSP.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Ghadially FN. Ultrastructural Pathology Of the Cell and Matrix: Butterworths-Heinemann, Boston; 1988.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

