Hypoxia augments outgrowth endothelial cell (OEC) sprouting and directed migration in response to sphingosine-1-phosphate (S1P)
- PMID: 25875493
- PMCID: PMC4398361
- DOI: 10.1371/journal.pone.0123437
Hypoxia augments outgrowth endothelial cell (OEC) sprouting and directed migration in response to sphingosine-1-phosphate (S1P)
Abstract
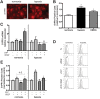
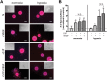
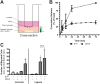
Therapeutic angiogenesis provides a promising approach to treat ischemic cardiovascular diseases through the delivery of proangiogenic cells and/or molecules. Outgrowth endothelial cells (OECs) are vascular progenitor cells that are especially suited for therapeutic strategies given their ease of noninvasive isolation from umbilical cord or adult peripheral blood and their potent ability to enhance tissue neovascularization. These cells are recruited to sites of vascular injury or tissue ischemia and directly incorporate within native vascular endothelium to participate in neovessel formation. A better understanding of how OEC activity may be boosted under hypoxia with external stimulation by proangiogenic molecules remains a challenge to improving their therapeutic potential. While vascular endothelial growth factor (VEGF) is widely established as a critical factor for initiating angiogenesis, sphingosine-1-phosphate (S1P), a bioactive lysophospholipid, has recently gained great enthusiasm as a potential mediator in neovascularization strategies. This study tests the hypothesis that hypoxia and the presence of VEGF impact the angiogenic response of OECs to S1P stimulation in vitro. We found that hypoxia altered the dynamically regulated S1P receptor 1 (S1PR1) expression on OECs in the presence of S1P (1.0 μM) and/or VEGF (1.3 nM). The combined stimuli of S1P and VEGF together promoted OEC angiogenic activity as assessed by proliferation, wound healing, 3D sprouting, and directed migration under both normoxia and hypoxia. Hypoxia substantially augmented the response to S1P alone, resulting in ~6.5-fold and ~25-fold increases in sprouting and directed migration, respectively. Overall, this report highlights the importance of establishing hypoxic conditions in vitro when studying ischemia-related angiogenic strategies employing vascular progenitor cells.
Conflict of interest statement
Figures


Similar articles
-
Alginate-Chitosan Hydrogels Provide a Sustained Gradient of Sphingosine-1-Phosphate for Therapeutic Angiogenesis.Ann Biomed Eng. 2017 Apr;45(4):1003-1014. doi: 10.1007/s10439-016-1768-2. Epub 2016 Nov 30. Ann Biomed Eng. 2017. PMID: 27904998 Free PMC article.
-
Alginate hydrogels of varied molecular weight distribution enable sustained release of sphingosine-1-phosphate and promote angiogenesis.J Biomed Mater Res A. 2018 Jan;106(1):138-146. doi: 10.1002/jbm.a.36217. Epub 2017 Sep 26. J Biomed Mater Res A. 2018. PMID: 28875559 Free PMC article.
-
Sphingosine signalling regulates decidual NK cell angiogenic phenotype and trophoblast migration.Hum Reprod. 2013 Nov;28(11):3026-37. doi: 10.1093/humrep/det339. Epub 2013 Sep 3. Hum Reprod. 2013. PMID: 24001716
-
Differential effects of sphingosine 1-phosphate and lysophosphatidic acid on endothelial cells.Biochim Biophys Acta. 2002 May 23;1582(1-3):190-6. doi: 10.1016/s1388-1981(02)00155-5. Biochim Biophys Acta. 2002. PMID: 12069828 Review.
-
Sphingosine-1-phosphate signaling and biological activities in the cardiovascular system.Biochim Biophys Acta. 2008 Sep;1781(9):483-8. doi: 10.1016/j.bbalip.2008.04.003. Epub 2008 Apr 22. Biochim Biophys Acta. 2008. PMID: 18472021 Review.
Cited by
-
S1PR1 as a Novel Promising Therapeutic Target in Cancer Therapy.Mol Diagn Ther. 2019 Aug;23(4):467-487. doi: 10.1007/s40291-019-00401-5. Mol Diagn Ther. 2019. PMID: 31115798 Review.
-
Alginate-Chitosan Hydrogels Provide a Sustained Gradient of Sphingosine-1-Phosphate for Therapeutic Angiogenesis.Ann Biomed Eng. 2017 Apr;45(4):1003-1014. doi: 10.1007/s10439-016-1768-2. Epub 2016 Nov 30. Ann Biomed Eng. 2017. PMID: 27904998 Free PMC article.
-
Lysophosphatidic Acid and Sphingosine-1-Phosphate: A Concise Review of Biological Function and Applications for Tissue Engineering.Tissue Eng Part B Rev. 2015 Dec;21(6):531-42. doi: 10.1089/ten.TEB.2015.0107. Epub 2015 Jul 14. Tissue Eng Part B Rev. 2015. PMID: 26035484 Free PMC article. Review.
-
Expansion of Sphingosine Kinase and Sphingosine-1-Phosphate Receptor Function in Normal and Cancer Cells: From Membrane Restructuring to Mediation of Estrogen Signaling and Stem Cell Programming.Int J Mol Sci. 2018 Jan 31;19(2):420. doi: 10.3390/ijms19020420. Int J Mol Sci. 2018. PMID: 29385066 Free PMC article. Review.
-
Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms.Prog Neurobiol. 2017 Nov;158:94-131. doi: 10.1016/j.pneurobio.2017.07.004. Epub 2017 Jul 23. Prog Neurobiol. 2017. PMID: 28743464 Free PMC article. Review.
References
-
- Fuchs S, Baffour R, Zhou YF, Shou M, Pierre A, Tio FO, et al. Transendocardial delivery of autologous bone marrow enhances collateral perfusion and regional function in pigs with chronic experimental myocardial ischemia. J Am Coll Cardiol. 2001. May;37(6):1726–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

