Macrophage PPARγ and impaired wound healing in type 2 diabetes
- PMID: 25875529
- PMCID: PMC4509817
- DOI: 10.1002/path.4548
Macrophage PPARγ and impaired wound healing in type 2 diabetes
Abstract
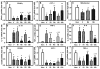
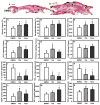
Macrophages undergo a transition from pro-inflammatory to healing-associated phenotypes that is critical for efficient wound healing. However, the regulation of this transition during normal and impaired healing remains to be elucidated. In our studies, the switch in macrophage phenotypes during skin wound healing was associated with up-regulation of the peroxisome proliferator-activated receptor (PPAR)γ and its downstream targets, along with increased mitochondrial content. In the setting of diabetes, up-regulation of PPARγ activity was impaired by sustained expression of IL-1β in both mouse and human wounds. In addition, experiments with myeloid-specific PPARγ knockout mice indicated that loss of PPARγ in macrophages is sufficient to prolong wound inflammation and delay healing. Furthermore, PPARγ agonists promoted a healing-associated macrophage phenotype both in vitro and in vivo, even in the diabetic wound environment. Importantly, topical administration of PPARγ agonists improved healing in diabetic mice, suggesting an appealing strategy for down-regulating inflammation and improving the healing of chronic wounds.
Keywords: diabetes; inflammation; macrophage; resolution of inflammation; wound healing.
Copyright © 2015 Pathological Society of Great Britain and Ireland. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Conflict of Interest Statement: The authors have no conflicting financial interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

