Soluble T-cell receptors produced in human cells for targeted delivery
- PMID: 25875651
- PMCID: PMC4395278
- DOI: 10.1371/journal.pone.0119559
Soluble T-cell receptors produced in human cells for targeted delivery
Abstract
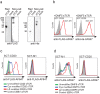
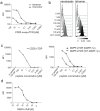
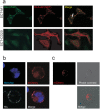
Recently, technology has become available to generate soluble T-cell receptors (sTCRs) that contain the antigen recognition part. In contrast to antibodies, sTCRs recognize intracellular in addition to extracellular epitopes, potentially increasing the number of applications as reagents for target detection and immunotherapy. Moreover, recent data show that they can be used for identification of their natural peptide ligands in disease. Here we describe a new and simplified expression method for sTCRs in human cells and show that these sTCRs can be used for antigen-specific labeling and elimination of human target cells. Four different TCRs were solubilized by expression of constructs encoding the TCR alpha (α) and beta (β) chains lacking the transmembrane and intracellular domains, linked by a ribosomal skipping 2A sequence that facilitates equimolar production of the chains. Cell supernatants containing sTCRs labeled target cells directly in a peptide (p)-human leukocyte antigen (HLA)-specific manner. We demonstrated that a MART-1p/HLA-A*02:01-specific sTCR fused to a fluorescent protein, or multimerized onto magnetic nanoparticles, could be internalized. Moreover, we showed that this sTCR and two sTCRs recognizing CD20p/HLA-A*02:01 could mediate selective elimination of target cells expressing the relevant pHLA complex when tetramerized to streptavidin-conjugated toxin, demonstrating the potential for specific delivery of cargo. This simple and efficient method can be utilized to generate a wide range of minimally modified sTCRs from the naturally occurring TCR repertoire for antigen-specific detection and targeting.
Conflict of interest statement
Figures
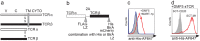

References
-
- Davis MM, Bjorkman PJ. T-cell antigen receptor genes and T-cell recognition. Nature. 1988;334(6181):395–402.. - PubMed
-
- Garcia KC, Degano M, Stanfield RL, Brunmark A, Jackson MR, Peterson PA, et al. An alphabeta T cell receptor structure at 2.5 A and its orientation in the TCR-MHC complex. Science. 1996;274(5285):209–19. . - PubMed
-
- Molloy PE, Sewell AK, Jakobsen BK. Soluble T cell receptors: novel immunotherapies. Curr Opin Pharmacol. 2005;5(4):438–43. . - PubMed
-
- Richman SA, Kranz DM. Display, engineering, and applications of antigen-specific T cell receptors. Biomol Eng. 2007;24(4):361–73. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

