Specific cellular incorporation of a pyrene-labelled cholesterol: lipoprotein-mediated delivery toward ordered intracellular membranes
- PMID: 25875769
- PMCID: PMC4398402
- DOI: 10.1371/journal.pone.0121563
Specific cellular incorporation of a pyrene-labelled cholesterol: lipoprotein-mediated delivery toward ordered intracellular membranes
Abstract
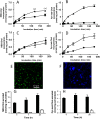
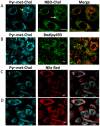
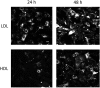
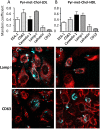
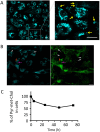
In the aim of testing tools for tracing cell trafficking of exogenous cholesterol, two fluorescent derivatives of cholesterol, 22-nitrobenzoxadiazole-cholesterol (NBD-Chol) and 21-methylpyrenyl-cholesterol (Pyr-met-Chol), with distinctive chemico-physical characteristics, have been compared for their cell incorporation properties, using two cell models differently handling cholesterol, with two incorporation routes. In the Caco-2 cell model, the cholesterol probes were delivered in bile salt micelles, as a model of intestinal absorption. The two probes displayed contrasting behaviors for cell uptake characteristics, cell staining, and efflux kinetics. In particular, Pyr-met-Chol cell incorporation involved SR-BI, while that of NBD-Chol appeared purely passive. In the PC-3 cell model, which overexpresses lipoprotein receptors, the cholesterol probes were delivered via the serum components, as a model of systemic delivery. We showed that Pyr-met-Chol-labelled purified LDL or HDL were able to specifically deliver Pyr-met-Chol to the PC-3 cells, while NBD-Chol incorporation was independent of lipoproteins. Observations by fluorescence microscopy evidenced that, while NBD-Chol readily stained the cytosolic lipid droplets, Pyr-met-Chol labelling led to the intense staining of intracellular structures of membranous nature, in agreement with the absence of detectable esterification of Pyr-met-Chol. A 48 h incubation of PC-3 cells with either Pyr-met-Chol-labelled LDL or HDL gave same staining patterns, mainly colocalizing with Lamp1, caveolin-1 and CD63. These data indicated convergent trafficking downwards their respective receptors, LDL-R and SR-BI, toward the cholesterol-rich internal membrane compartments, late endosomes and multivesicular bodies. Interestingly, Pyr-met-Chol staining of these structures exhibited a high excimer fluorescence emission, revealing their ordered membrane environment, and indicating that Pyr-met-Chol behaves as a fair cholesterol tracer regarding its preferential incorporation into cholesterol-rich domains. We conclude that, while NBD-Chol is a valuable marker of cholesterol esterification, Pyr-met-Chol is a reliable new lipoprotein fluorescent marker which allows to probe specific intracellular trafficking of cholesterol-rich membranes.
Conflict of interest statement
Figures


References
-
- Simons K, Ikonen E (2000) How cells handle cholesterol. Science 290: 1721–1726. - PubMed
-
- Soccio RE, Breslow JL (2004) Intracellular cholesterol transport. Arterioscler Thromb Vasc Biol 24: 1150–1160. - PubMed
-
- Hofnagel O, Luechtenborg B, Weissen-Plenz G, Robenek H (2007) Statins and foam cell formation: impact on LDL oxidation and uptake of oxidized lipoproteins via scavenger receptors. Biochim Biophys Acta 1771: 1117–1124. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

