The clinical effect of deferoxamine mesylate on edema after intracerebral hemorrhage
- PMID: 25875777
- PMCID: PMC4395224
- DOI: 10.1371/journal.pone.0122371
The clinical effect of deferoxamine mesylate on edema after intracerebral hemorrhage
Abstract
Background and purpose: It has been shown that 3 days of 62 mg/kg/day deferoxamine infusion (maximum dose not to exceed 6000 mg/day) is safe and tolerated by intracerebral hemorrhage (ICH) patients. The aim of this study was to investigate the efficacy of deferoxamine mesylate for edema resolution and hematoma absorption after ICH.
Methods: From February 2013 to May 2014, spontaneous ICH patients diagnosed by computed tomography (CT) within 18 hours of onset were evaluated. Patients were randomly divided into two groups: an experimental group and a control group. The treatment of the two groups was similar except that the experimental group received deferoxamine mesylate. Patients were evaluated by CT and neurology scale at the time of admission, and on the fourth, eighth, and fifteenth day (or at discharge) after admission. Patients were followed up for the first 30 days and clinical data of the two groups were compared.
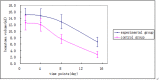
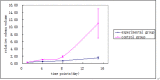
Results: Forty-two patients completed 30 days of follow-up by May 2014; 21 cases in the experimental group and 21 cases in the control group. The control group's relative edema volume on the fifteenth day (or discharge) was 10.26 ± 17.54, which was higher than the experimental group (1.91 ± 1.94; P < 0.05). The control group's 1-8 day and 8-15 day relative hematoma absorption were greater than the experimental group (P < 0.05).The control group's relative edema volume on the fourth, eighth, and fifteenth day (or discharge) was higher than the experimental group (P < 0.05). Neurological scores between the two groups were not statistically different on the fifteenth day (or discharge) or on the thirtieth day.
Conclusions: Deferoxamine mesylate may slow hematoma absorption and inhibit edema after ICH, although further investigation is required to form definitive conclusions.
Trial registration: Chinese Clinical Trial Registry ChiCTR-TRC-14004979.
Conflict of interest statement
Figures
References
-
- Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2004; 2:43–53. - PubMed
-
- Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol.2006; 5: 53–63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources