Primary and liver metastasis-derived cell lines from KrasG12D; Trp53R172H; Pdx-1 Cre animals undergo apoptosis in response to triptolide
- PMID: 25875797
- PMCID: PMC4412369
- DOI: 10.1097/MPA.0000000000000317
Primary and liver metastasis-derived cell lines from KrasG12D; Trp53R172H; Pdx-1 Cre animals undergo apoptosis in response to triptolide
Abstract
Objectives: Pancreatic cancer has a 5-year survival rate of less than 5%, partly because of limited chemotherapeutic options, thereby highlighting the need for novel therapies. Triptolide, a diterpene triepoxide that was derived from a Chinese herb, has shown great promise in preclinical testing against pancreatic cancer using immunocompromised animals.
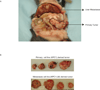
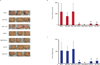
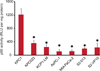
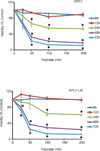
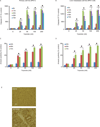
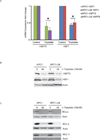
Results: In this study, we tested the ability of triptolide to induce cell death in cell lines derived from a primary tumor and adjacent liver metastases of immunocompetent animals (Kras, Trp53, Pdx-1 Cre [KPC]). Both cell lines were more aggressive in their ability to form tumors when compared with other pancreatic cancer cell lines and showed constitutive activation of the nuclear factor κ-light-chain-enhancer of activated B cells pathway. Triptolide induced apoptotic cell death in both cell lines, as evidenced by decreased cell viability as well as increased caspase 3/7 activity, annexin V positivity, and increased terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling positivity in tumors from KPC animals treated with Minnelide. In addition, triptolide decreased levels of HSP70, its transcription factor HSF1, as well as the antiapoptotic proteins Bcl-xL, Bcl-2, and Mcl-1, which are known to be up-regulated in pancreatic cancer.
Conclusions: The ability of triptolide to cause cell death in cell lines derived from immunocompetent animals further validates its potential as a novel agent against pancreatic cancer.
Figures
Similar articles
-
Triptolide induces pancreatic cancer cell death via inhibition of heat shock protein 70.Cancer Res. 2007 Oct 1;67(19):9407-16. doi: 10.1158/0008-5472.CAN-07-1077. Cancer Res. 2007. PMID: 17909050
-
Triptolide as a novel agent in pancreatic cancer: the validation using patient derived pancreatic tumor cell line.BMC Cancer. 2018 Nov 12;18(1):1103. doi: 10.1186/s12885-018-4995-0. BMC Cancer. 2018. PMID: 30419860 Free PMC article.
-
Role of Hsp-70 in triptolide-mediated cell death of neuroblastoma.J Surg Res. 2010 Sep;163(1):72-8. doi: 10.1016/j.jss.2010.04.047. Epub 2010 May 20. J Surg Res. 2010. PMID: 20638672 Free PMC article.
-
Triptolide and Its Derivatives as Cancer Therapies.Trends Pharmacol Sci. 2019 May;40(5):327-341. doi: 10.1016/j.tips.2019.03.002. Epub 2019 Apr 8. Trends Pharmacol Sci. 2019. PMID: 30975442 Review.
-
Minnelide, a novel drug for pancreatic and liver cancer.Pancreatology. 2015 Jul;15(4 Suppl):S39-43. doi: 10.1016/j.pan.2015.05.472. Epub 2015 Jun 18. Pancreatology. 2015. PMID: 26122306 Free PMC article. Review.
Cited by
-
Reprogramming the Tolerogenic Immune Response Against Pancreatic Cancer Metastases by Lipid Nanoparticles Delivering a STING Agonist Plus Mutant KRAS mRNA.ACS Nano. 2025 Mar 11;19(9):8579-8594. doi: 10.1021/acsnano.4c14102. Epub 2025 Mar 2. ACS Nano. 2025. PMID: 40025875 Free PMC article.
-
Bioactive Compounds: Natural Defense Against Cancer?Biomolecules. 2019 Nov 21;9(12):758. doi: 10.3390/biom9120758. Biomolecules. 2019. PMID: 31766399 Free PMC article. Review.
-
Targeting tumor-intrinsic hexosamine biosynthesis sensitizes pancreatic cancer to anti-PD1 therapy.J Clin Invest. 2020 Jan 2;130(1):451-465. doi: 10.1172/JCI127515. J Clin Invest. 2020. PMID: 31613799 Free PMC article.
-
LSL-KrasG12D; LSL-Trp53R172H/+; Ink4flox/+; Ptf1/p48-Cre mice are an applicable model for locally invasive and metastatic pancreatic cancer.PLoS One. 2017 May 5;12(5):e0176844. doi: 10.1371/journal.pone.0176844. eCollection 2017. PLoS One. 2017. PMID: 28475592 Free PMC article.
-
All trans-retinoic acid analogs promote cancer cell apoptosis through non-genomic Crabp1 mediating ERK1/2 phosphorylation.Sci Rep. 2016 Mar 3;6:22396. doi: 10.1038/srep22396. Sci Rep. 2016. PMID: 26935534 Free PMC article.
References
-
- Warshaw AL, Gu ZY, Wittenberg J, et al. Preoperative staging and assessment of resectability of pancreatic cancer. Arch Surg. 1990;125:230–233. - PubMed
-
- Burris H, Storniolo AM. Assessing clinical benefit in the treatment of pancreas cancer: gemcitabine compared to 5-fluorouracil. Eur J Cancer. 1997;33(Suppl 1):S18–S22. - PubMed
-
- Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. - PubMed
-
- Phillips PA, Dudeja V, McCarroll JA, et al. Triptolide induces pancreatic cancer cell death via inhibition of heat shock protein 70. Cancer Res. 2007;67:9407–9416. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

