A missense change in the ATG4D gene links aberrant autophagy to a neurodegenerative vacuolar storage disease
- PMID: 25875846
- PMCID: PMC4398399
- DOI: 10.1371/journal.pgen.1005169
A missense change in the ATG4D gene links aberrant autophagy to a neurodegenerative vacuolar storage disease
Abstract
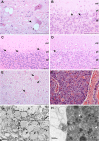
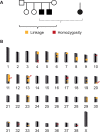
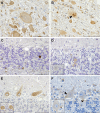
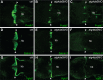
Inherited neurodegenerative disorders are debilitating diseases that occur across different species. We have performed clinical, pathological and genetic studies to characterize a novel canine neurodegenerative disease present in the Lagotto Romagnolo dog breed. Affected dogs suffer from progressive cerebellar ataxia, sometimes accompanied by episodic nystagmus and behavioral changes. Histological examination revealed unique pathological changes, including profound neuronal cytoplasmic vacuolization in the nervous system, as well as spheroid formation and cytoplasmic aggregation of vacuoles in secretory epithelial tissues and mesenchymal cells. Genetic analyses uncovered a missense change, c.1288G>A; p.A430T, in the autophagy-related ATG4D gene on canine chromosome 20 with a highly significant disease association (p = 3.8 x 10-136) in a cohort of more than 2300 Lagotto Romagnolo dogs. ATG4D encodes a poorly characterized cysteine protease belonging to the macroautophagy pathway. Accordingly, our histological analyses indicated altered autophagic flux in affected tissues. The knockdown of the zebrafish homologue atg4da resulted in a widespread developmental disturbance and neurodegeneration in the central nervous system. Our study describes a previously unknown canine neurological disease with particular pathological features and implicates the ATG4D protein as an important autophagy mediator in neuronal homeostasis. The canine phenotype serves as a model to delineate the disease-causing pathological mechanism(s) and ATG4D function, and can also be used to explore treatment options. Furthermore, our results reveal a novel candidate gene for human neurodegeneration and enable the development of a genetic test for veterinary diagnostic and breeding purposes.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: A genetic test will be available later from Genoscoper Ltd, which is partly owned by HL.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

