Metabolic plasticity in resting and thrombin activated platelets
- PMID: 25875958
- PMCID: PMC4395425
- DOI: 10.1371/journal.pone.0123597
Metabolic plasticity in resting and thrombin activated platelets
Abstract
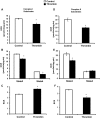
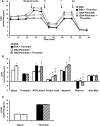
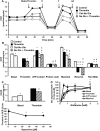
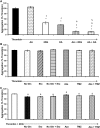
Platelet thrombus formation includes several integrated processes involving aggregation, secretion of granules, release of arachidonic acid and clot retraction, but it is not clear which metabolic fuels are required to support these events. We hypothesized that there is flexibility in the fuels that can be utilized to serve the energetic and metabolic needs for resting and thrombin-dependent platelet aggregation. Using platelets from healthy human donors, we found that there was a rapid thrombin-dependent increase in oxidative phosphorylation which required both glutamine and fatty acids but not glucose. Inhibition of fatty acid oxidation or glutamine utilization could be compensated for by increased glycolytic flux. No evidence for significant mitochondrial dysfunction was found, and ATP/ADP ratios were maintained following the addition of thrombin, indicating the presence of functional and active mitochondrial oxidative phosphorylation during the early stages of aggregation. Interestingly, inhibition of fatty acid oxidation and glutaminolysis alone or in combination is not sufficient to prevent platelet aggregation, due to compensation from glycolysis, whereas inhibitors of glycolysis inhibited aggregation approximately 50%. The combined effects of inhibitors of glycolysis and oxidative phosphorylation were synergistic in the inhibition of platelet aggregation. In summary, both glycolysis and oxidative phosphorylation contribute to platelet metabolism in the resting and activated state, with fatty acid oxidation and to a smaller extent glutaminolysis contributing to the increased energy demand.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

