Interfacial pH during mussel adhesive plaque formation
- PMID: 25875963
- PMCID: PMC4420479
- DOI: 10.1080/08927014.2015.1026337
Interfacial pH during mussel adhesive plaque formation
Abstract
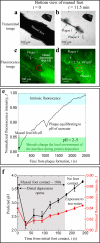
Mussel (Mytilus californianus) adhesion to marine surfaces involves an intricate and adaptive synergy of molecules and spatio-temporal processes. Although the molecules, such as mussel foot proteins (mfps), are well characterized, deposition details remain vague and speculative. Developing methods for the precise surveillance of conditions that apply during mfp deposition would aid both in understanding mussel adhesion and translating this adhesion into useful technologies. To probe the interfacial pH at which mussels buffer the local environment during mfp deposition, a lipid bilayer with tethered pH-sensitive fluorochromes was assembled on mica. The interfacial pH during foot contact with modified mica ranged from 2.2 to 3.3, which is well below the seawater pH of ~ 8. The acidic pH serves multiple functions: it limits mfp-Dopa oxidation, thereby enabling the catecholic functionalities to adsorb to surface oxides by H-bonding and metal ion coordination, and provides a solubility switch for mfps, most of which aggregate at pH ≥ 7-8.
Keywords: Dopa; Oregon Green® 488 DHPE; mussel interfacial pH; pH sensitive surface.
Figures


References
-
- Ahn BK, Lee DW, Israelachvili JN, Waite JH. Surface-initiated self-healing of polymers in aqueous media. Nature materials. 2014;13:867–872. - PubMed
-
- Carrington E, Waite JH, Sarà G, Sebens KP. Mussels as a model system for integrative ecomechanics. Marine Science. 2015;7 - PubMed
-
- Dorsett LC, Hawkins CJ, Grice JA, Lavin MF, Merefield PM, Parry DL, Ross IL. Ferreascidin: a highly aromatic protein containing 3, 4-dihydroxyphenylalanine from the blood cells of a stolidobranch ascidian. Biochemistry. 1987;26:8078–8082.
-
- Draper NR, Smith H, Pownell E. Applied regression analysis. Wiley; New York: 1966.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
