Elucidating the crucial role of poly N-acetylglucosamine from Staphylococcus aureus in cellular adhesion and pathogenesis
- PMID: 25876106
- PMCID: PMC4398431
- DOI: 10.1371/journal.pone.0124216
Elucidating the crucial role of poly N-acetylglucosamine from Staphylococcus aureus in cellular adhesion and pathogenesis
Abstract
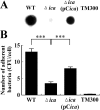
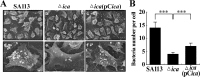
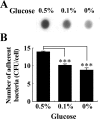
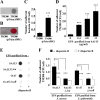
Staphylococcus aureus is an important pathogen that forms biofilms on the surfaces of medical implants. Biofilm formation by S. aureus is associated with the production of poly N-acetylglucosamine (PNAG), also referred to as polysaccharide intercellular adhesin (PIA), which mediates bacterial adhesion, leading to the accumulation of bacteria on solid surfaces. This study shows that the ability of S. aureus SA113 to adhere to nasal epithelial cells is reduced after the deletion of the ica operon, which contains genes encoding PIA/PNAG synthesis. However, this ability is restored after a plasmid carrying the entire ica operon is transformed into the mutant strain, S. aureus SA113Δica, showing that the synthesis of PIA/PNAG is important for adhesion to epithelial cells. Additionally, S. carnosus TM300, which does not produce PIA/PNAG, forms a biofilm and adheres to epithelial cells after the bacteria are transformed with a PIA/PNAG-expressing plasmid, pTXicaADBC. The adhesion of S. carnosus TM300 to epithelial cells is also demonstrated by adding purified exopolysaccharide (EPS), which contains PIA/PNAG, to the bacteria. In addition, using a mouse model, we find that the abscess lesions and bacterial burden in lung tissues is higher in mice infected with S. aureus SA113 than in those infected with the mutant strain, S. aureus SA113Δica. The results indicate that PIA/PNAG promotes the adhesion of S. aureus to human nasal epithelial cells and lung infections in a mouse model. This study elucidates a mechanism that is important to the pathogenesis of S. aureus infections.
Conflict of interest statement
Figures
Similar articles
-
σB Inhibits Poly-N-Acetylglucosamine Exopolysaccharide Synthesis and Biofilm Formation in Staphylococcus aureus.J Bacteriol. 2019 May 8;201(11):e00098-19. doi: 10.1128/JB.00098-19. Print 2019 Jun 1. J Bacteriol. 2019. PMID: 30858304 Free PMC article.
-
Phase variation of poly-N-acetylglucosamine expression in Staphylococcus aureus.PLoS Pathog. 2014 Jul 31;10(7):e1004292. doi: 10.1371/journal.ppat.1004292. eCollection 2014 Jul. PLoS Pathog. 2014. PMID: 25077798 Free PMC article.
-
A Novel Repressor of the ica Locus Discovered in Clinically Isolated Super-Biofilm-Elaborating Staphylococcus aureus.mBio. 2017 Jan 31;8(1):e02282-16. doi: 10.1128/mBio.02282-16. mBio. 2017. PMID: 28143981 Free PMC article.
-
Polysaccharide intercellular adhesin in biofilm: structural and regulatory aspects.Front Cell Infect Microbiol. 2015 Feb 10;5:7. doi: 10.3389/fcimb.2015.00007. eCollection 2015. Front Cell Infect Microbiol. 2015. PMID: 25713785 Free PMC article. Review.
-
ica and beyond: biofilm mechanisms and regulation in Staphylococcus epidermidis and Staphylococcus aureus.FEMS Microbiol Lett. 2007 May;270(2):179-88. doi: 10.1111/j.1574-6968.2007.00688.x. Epub 2007 Apr 10. FEMS Microbiol Lett. 2007. PMID: 17419768 Review.
Cited by
-
Targeted bacterial conjugation mediated by synthetic cell-to-cell adhesions.Nucleic Acids Res. 2022 Dec 9;50(22):12938-12950. doi: 10.1093/nar/gkac1164. Nucleic Acids Res. 2022. PMID: 36511856 Free PMC article.
-
Spatial organization of the kelp microbiome at micron scales.Microbiome. 2022 Mar 24;10(1):52. doi: 10.1186/s40168-022-01235-w. Microbiome. 2022. PMID: 35331334 Free PMC article.
-
Extended-spectrum antibodies protective against carbapenemase-producing Enterobacteriaceae.J Antimicrob Chemother. 2016 Apr;71(4):927-35. doi: 10.1093/jac/dkv448. Epub 2016 Jan 7. J Antimicrob Chemother. 2016. PMID: 26747103 Free PMC article.
-
Mycoplasma genitalium Biofilms Contain Poly-GlcNAc and Contribute to Antibiotic Resistance.Front Microbiol. 2020 Oct 27;11:585524. doi: 10.3389/fmicb.2020.585524. eCollection 2020. Front Microbiol. 2020. PMID: 33193233 Free PMC article.
-
Within-Host Adaptation of Staphylococcus aureus in a Bovine Mastitis Infection Is Associated with Increased Cytotoxicity.Int J Mol Sci. 2021 Aug 17;22(16):8840. doi: 10.3390/ijms22168840. Int J Mol Sci. 2021. PMID: 34445550 Free PMC article.
References
-
- Götz F. Staphylococci in colonization and disease: prospective targets for drugs and vaccines. Curr Opin Microbiol. 2004; 7: 477–487. - PubMed
-
- Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005; 5: 751–762. - PubMed
-
- Wertheim HF, Vos MC, Ott A, van Belkum A, Voss A, Kluytmans JA, et al. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet. 2004; 364: 703–705. - PubMed
-
- Götz F. Staphylococcus and biofilms. Mol Microbiol. 2002; 43: 1367–1378. - PubMed
-
- Gerke C, Kraft A, Sussmuth R, Schweitzer O, Götz F. Characterization of the N-acetylglucosaminyltransferase activity involved in the biosynthesis of the Staphylococcus epidermidis polysaccharide intercellular adhesin. J Biol Chem. 1998; 273: 18586–18593. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

