Progress on adenovirus-vectored universal influenza vaccines
- PMID: 25876176
- PMCID: PMC4514376
- DOI: 10.1080/21645515.2015.1016674
Progress on adenovirus-vectored universal influenza vaccines
Abstract
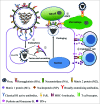
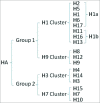
Influenza virus (IFV) infection causes serious health problems and heavy financial burdens each year worldwide. The classical inactivated influenza virus vaccine (IIVV) and live attenuated influenza vaccine (LAIV) must be updated regularly to match the new strains that evolve due to antigenic drift and antigenic shift. However, with the discovery of broadly neutralizing antibodies that recognize conserved antigens, and the CD8(+) T cell responses targeting viral internal proteins nucleoprotein (NP), matrix protein 1 (M1) and polymerase basic 1 (PB1), it is possible to develop a universal influenza vaccine based on the conserved hemagglutinin (HA) stem, NP, and matrix proteins. Recombinant adenovirus (rAd) is an ideal influenza vaccine vector because it has an ideal stability and safety profile, induces balanced humoral and cell-mediated immune responses due to activation of innate immunity, provides 'self-adjuvanting' activity, can mimic natural IFV infection, and confers seamless protection against mucosal pathogens. Moreover, this vector can be developed as a low-cost, rapid-response vaccine that can be quickly manufactured. Therefore, an adenovirus vector encoding conserved influenza antigens holds promise in the development of a universal influenza vaccine. This review will summarize the progress in adenovirus-vectored universal flu vaccines and discuss future novel approaches.
Keywords: ADCC, antibody-dependent cell-mediated cytotoxicity; APC, antigen-presenting cell; Ad: adenovirus; CAR, Coxsackie-Adenovirus Receptor; CTLs, cytotoxic T lymphocytes; DC, lung dendritic cells; DVD, drug–vaccine duo; FcγRs, Fc receptors for IgG; HA, hemagglutinin; HDAd, helper-dependent adenoviral; HEK293, human embryonic kidney 293 cell; HI, hemagglutination inhibition; HLA, human leukocyte antigen; IF-γ, interferon-γ; IFV, Influenza virus; IIVV, inactivated influenza virus vaccine; IL-2, interleukin-2; ITRs, inverted terminal repeats; LAIV, live attenuated influenza vaccine; M1, matrix protein 1; M2, matrix protein 2; MHC-I, major histocompatibility complex class I; NA, neuraminidase; NP, nucleoprotein; RCA, replication competent adenovirus; VAERD, vaccine-associated enhanced respiratory disease; adenovirus vector; broadly neutralizing antibodies; cellular immunity; flu, influenza; hemagglutinin; humoral immunity; influenza; mAbs, monoclonal antibodies; mucosal immunity; rAd, recombinant adenovirus; universal vaccine.
Figures
Similar articles
-
Intranasal adenovirus-vectored vaccine for induction of long-lasting humoral immunity-mediated broad protection against influenza in mice.J Virol. 2014 Sep 1;88(17):9693-703. doi: 10.1128/JVI.00823-14. Epub 2014 Jun 11. J Virol. 2014. PMID: 24920793 Free PMC article.
-
Protective Efficacy of the Conserved NP, PB1, and M1 Proteins as Immunogens in DNA- and Vaccinia Virus-Based Universal Influenza A Virus Vaccines in Mice.Clin Vaccine Immunol. 2015 Jun;22(6):618-30. doi: 10.1128/CVI.00091-15. Epub 2015 Apr 1. Clin Vaccine Immunol. 2015. PMID: 25834017 Free PMC article.
-
Cross-protective efficacy and safety of an adenovirus-based universal influenza vaccine expressing nucleoprotein, hemagglutinin, and the ectodomain of matrix protein 2.Vaccine. 2024 May 31;42(15):3505-3513. doi: 10.1016/j.vaccine.2024.04.054. Epub 2024 May 6. Vaccine. 2024. PMID: 38714444
-
Virus-vectored influenza virus vaccines.Viruses. 2014 Aug 7;6(8):3055-79. doi: 10.3390/v6083055. Viruses. 2014. PMID: 25105278 Free PMC article. Review.
-
Development of universal influenza vaccines based on influenza virus M and NP genes.Infection. 2014 Apr;42(2):251-62. doi: 10.1007/s15010-013-0546-4. Epub 2013 Nov 1. Infection. 2014. PMID: 24178189 Review.
Cited by
-
The immunological impact of adenovirus early genes on vaccine-induced responses in mice and nonhuman primates.J Virol. 2021 Mar 10;95(7):e02253-20. doi: 10.1128/JVI.02253-20. Epub 2021 Jan 13. J Virol. 2021. PMID: 33441339 Free PMC article.
-
Increased humoral immunity by DNA vaccination using an α-tocopherol-based adjuvant.Hum Vaccin Immunother. 2017 Aug 3;13(8):1823-1830. doi: 10.1080/21645515.2017.1321183. Epub 2017 Jun 14. Hum Vaccin Immunother. 2017. PMID: 28613978 Free PMC article.
-
Development of a perfusion process for serum-free adenovirus vector herpes zoster vaccine production.AMB Express. 2022 May 14;12(1):58. doi: 10.1186/s13568-022-01398-7. AMB Express. 2022. PMID: 35567723 Free PMC article.
-
Vaccination potential of B and T epitope-enriched NP and M2 against Influenza A viruses from different clades and hosts.PLoS One. 2018 Jan 29;13(1):e0191574. doi: 10.1371/journal.pone.0191574. eCollection 2018. PLoS One. 2018. PMID: 29377916 Free PMC article.
-
Plasma cell and serum antibody responses to influenza vaccine in preterm and full-term infants.Vaccine. 2017 Sep 12;35(38):5163-5171. doi: 10.1016/j.vaccine.2017.07.115. Epub 2017 Aug 12. Vaccine. 2017. PMID: 28807607 Free PMC article.
References
-
- WHO Influenza (Seasonal). Disponibile al link: http://wwwwhoint/mediacentre/factsheets/fs211/en/, 2014
-
- WHO Influenza update. http://wwwwhoint/influenza/surveillance_monitoring/updates/latest_update..., 2014
-
- Lagace-Wiens PR, Rubinstein E, Gumel A. Influenza epidemiology–past, present, and future. Crit Care Med 2010; 38:e1-9; PMID:20029350; http://dx.doi.org/10.1097/CCM.0b013e3181cbaf34 - DOI - PubMed
-
- Tong S, Li Y, Rivailler P, Conrardy C, Castillo DA, Chen LM, Recuenco S, Ellison JA, Davis CT, York IA, et al.. A distinct lineage of influenza A virus from bats. Proc Natl Acad Sci U S A 2012; 109:4269-74; PMID:22371588; http://dx.doi.org/10.1073/pnas.1116200109 - DOI - PMC - PubMed
-
- Emanuel EJ, Wertheimer A. Who should get influenza vaccine when not all can? Public Health Ethik 2010; 1:191.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
