Progress on adenovirus-vectored universal influenza vaccines
- PMID: 25876176
- PMCID: PMC4514376
- DOI: 10.1080/21645515.2015.1016674
Progress on adenovirus-vectored universal influenza vaccines
Abstract
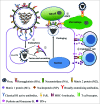
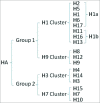
Influenza virus (IFV) infection causes serious health problems and heavy financial burdens each year worldwide. The classical inactivated influenza virus vaccine (IIVV) and live attenuated influenza vaccine (LAIV) must be updated regularly to match the new strains that evolve due to antigenic drift and antigenic shift. However, with the discovery of broadly neutralizing antibodies that recognize conserved antigens, and the CD8(+) T cell responses targeting viral internal proteins nucleoprotein (NP), matrix protein 1 (M1) and polymerase basic 1 (PB1), it is possible to develop a universal influenza vaccine based on the conserved hemagglutinin (HA) stem, NP, and matrix proteins. Recombinant adenovirus (rAd) is an ideal influenza vaccine vector because it has an ideal stability and safety profile, induces balanced humoral and cell-mediated immune responses due to activation of innate immunity, provides 'self-adjuvanting' activity, can mimic natural IFV infection, and confers seamless protection against mucosal pathogens. Moreover, this vector can be developed as a low-cost, rapid-response vaccine that can be quickly manufactured. Therefore, an adenovirus vector encoding conserved influenza antigens holds promise in the development of a universal influenza vaccine. This review will summarize the progress in adenovirus-vectored universal flu vaccines and discuss future novel approaches.
Keywords: ADCC, antibody-dependent cell-mediated cytotoxicity; APC, antigen-presenting cell; Ad: adenovirus; CAR, Coxsackie-Adenovirus Receptor; CTLs, cytotoxic T lymphocytes; DC, lung dendritic cells; DVD, drug–vaccine duo; FcγRs, Fc receptors for IgG; HA, hemagglutinin; HDAd, helper-dependent adenoviral; HEK293, human embryonic kidney 293 cell; HI, hemagglutination inhibition; HLA, human leukocyte antigen; IF-γ, interferon-γ; IFV, Influenza virus; IIVV, inactivated influenza virus vaccine; IL-2, interleukin-2; ITRs, inverted terminal repeats; LAIV, live attenuated influenza vaccine; M1, matrix protein 1; M2, matrix protein 2; MHC-I, major histocompatibility complex class I; NA, neuraminidase; NP, nucleoprotein; RCA, replication competent adenovirus; VAERD, vaccine-associated enhanced respiratory disease; adenovirus vector; broadly neutralizing antibodies; cellular immunity; flu, influenza; hemagglutinin; humoral immunity; influenza; mAbs, monoclonal antibodies; mucosal immunity; rAd, recombinant adenovirus; universal vaccine.
Figures
References
-
- WHO Influenza (Seasonal). Disponibile al link: http://wwwwhoint/mediacentre/factsheets/fs211/en/, 2014
-
- WHO Influenza update. http://wwwwhoint/influenza/surveillance_monitoring/updates/latest_update..., 2014
-
- Lagace-Wiens PR, Rubinstein E, Gumel A. Influenza epidemiology–past, present, and future. Crit Care Med 2010; 38:e1-9; PMID:20029350; http://dx.doi.org/ 10.1097/CCM.0b013e3181cbaf34 - DOI - PubMed
-
- Tong S, Li Y, Rivailler P, Conrardy C, Castillo DA, Chen LM, Recuenco S, Ellison JA, Davis CT, York IA, et al.. A distinct lineage of influenza A virus from bats. Proc Natl Acad Sci U S A 2012; 109:4269-74; PMID:22371588; http://dx.doi.org/ 10.1073/pnas.1116200109 - DOI - PMC - PubMed
-
- Emanuel EJ, Wertheimer A. Who should get influenza vaccine when not all can? Public Health Ethik 2010; 1:191.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
