MiR-27a ameliorates inflammatory damage to the blood-spinal cord barrier after spinal cord ischemia: reperfusion injury in rats by downregulating TICAM-2 of the TLR4 signaling pathway
- PMID: 25876455
- PMCID: PMC4336736
- DOI: 10.1186/s12974-015-0246-3
MiR-27a ameliorates inflammatory damage to the blood-spinal cord barrier after spinal cord ischemia: reperfusion injury in rats by downregulating TICAM-2 of the TLR4 signaling pathway
Abstract
Background: Spinal cord ischemia reperfusion (IR) injury causes inflammation and subsequently increases blood-spinal cord barrier leakage and Toll-like receptor 4 (TLR4) pathway activation. MicroRNAs (miRs) effectively regulate numerous target mRNAs during ischemia. However, their roles during IR injury are poorly understood. We investigated miRs involvement, particularly miR-27a, in TLR4 pathway-mediated inflammatory responses after IR.
Method: We used a genomics approach to examine changed miRs of rats that had undergone 14 minutes of ischemia, followed by 24 or 72 hours of reperfusion. Quantitative RT-PCR was used to identify and confirm the miRs involved in regulating TLR4 pathway activation. We scanned miR databases for potential miR targets and confirmed these targets by quantitative RT-PCR. The miR mimic and anti-miR oligonucleotides (AMOs) were intrathecally injected at 12-hour intervals beginning three days before the ischemia. The effects of miRs on the TLR4 pathway and downstream cytokines were analyzed by PCR, western blotting, and ELISA. Double immunofluorescence staining was perfumed to determine the relationship between the targets and TLR4. Blood-spinal cord barrier (BSCB) permeability was examined using Evans blue (EB) dye.
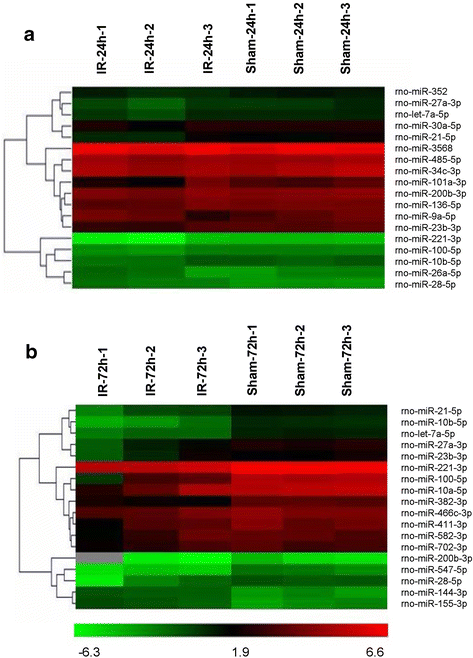
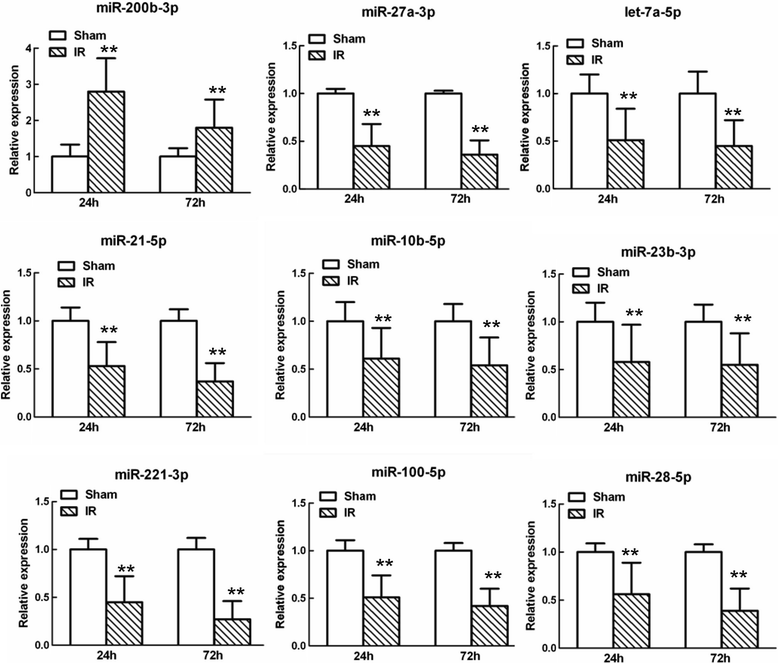
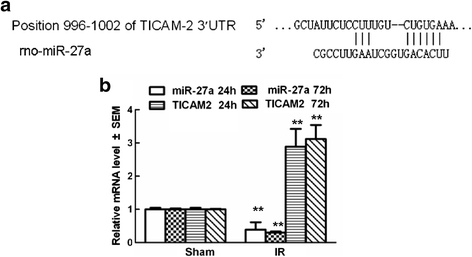
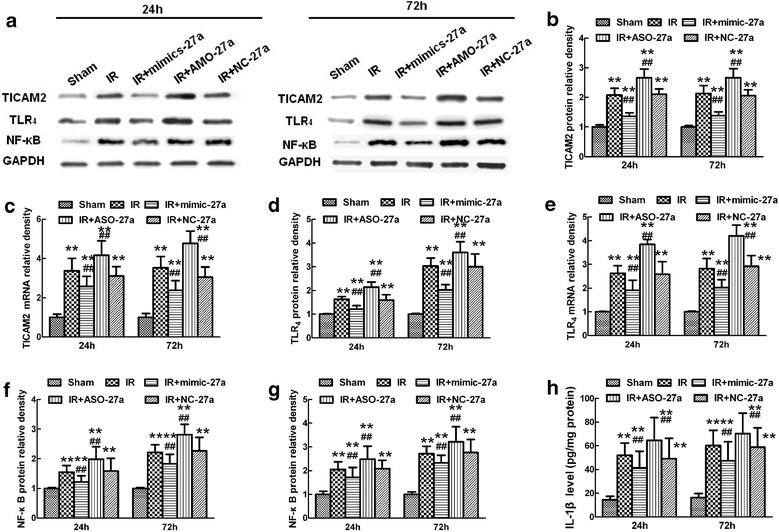
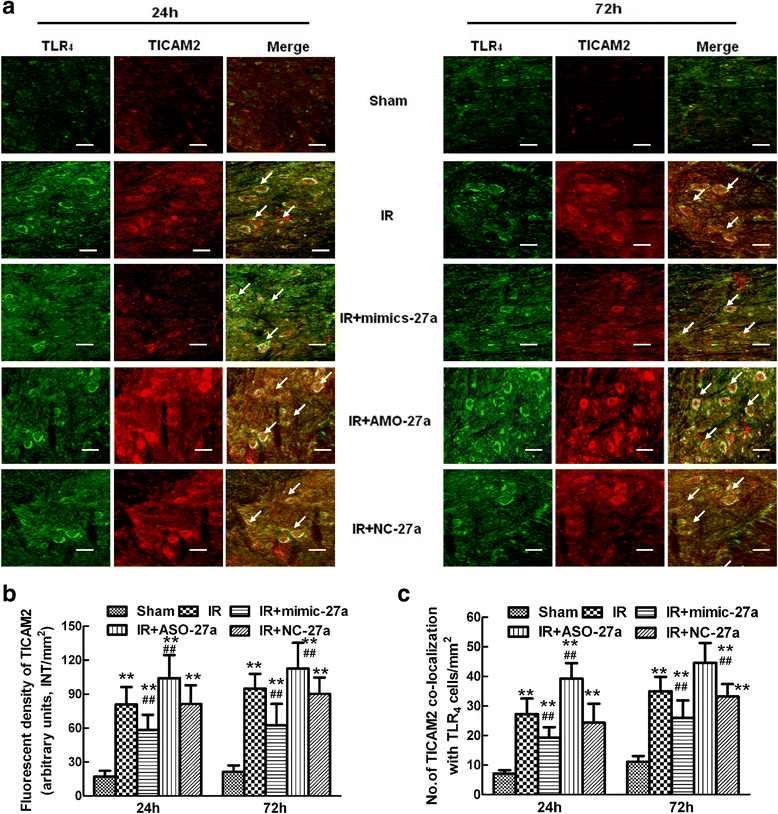
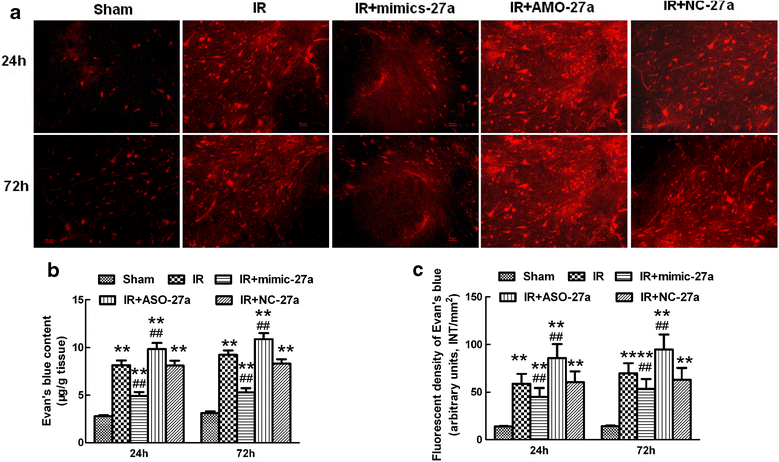
Results: A microarray analysis revealed that at 24 hours post-injury, three miRs were upregulated (>2.0 fold) and 15 miRs were downregulated (<0.5 fold), and at 72 hours, four miRs were upregulated and 14 were downregulated compared to their levels in sham-operated controls. We focused on miR-27a, which is predicted to contain sequences complementary to the 3'-untranslated region (UTR) of Toll-like receptor adaptor molecule 2 (TICAM-2). Double immunostaining indicated that TLR4 activation correlated with changes in TICAM-2 expression. Compared to the rats in the IR and negative control groups, intrathecal infusion of the miR-27a mimic attenuated IR-induced TLR4 activation and inflammatory damage to the BSCB, which was shown as decreased EB extravasation and lower levels of nuclear factor kappa-B (NF-κB) and lnterleukin (IL)-1β at 24 and 72 hours after reperfusion, whereas pretreatment with miR-27a AMO aggravated these injuries.
Conclusions: We present the first evidence that miRs play an important role in spinal cord IR injury. We identified TICAM-2 as a novel target of miR-27a. miR-27a upregulation attenuates IR-induced inflammatory damage to the BSCB by negatively regulating TICAM-2 of the TLR4 signaling pathway and inhibiting the NF-κB/IL-1β pathway. These results provide new therapeutic targets for IR injury treatment.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

