Urine lipoarabinomannan to monitor antituberculosis therapy response and predict mortality in an HIV-endemic region: a prospective cohort study
- PMID: 25877271
- PMCID: PMC4401837
- DOI: 10.1136/bmjopen-2014-006833
Urine lipoarabinomannan to monitor antituberculosis therapy response and predict mortality in an HIV-endemic region: a prospective cohort study
Abstract
Objective: To determine if urinary lipoarabinomannan (LAM) may serve as a biomarker to monitor antituberculosis (TB) therapy response, and whether LAM results before and after treatment are predictive of patient outcomes.
Design: Prospective cohort.
Setting: Outpatient referral clinic and tertiary hospital in South Africa.
Participants: Adults (≥18 years) with ≥2 TB-related symptoms (cough, fever, weight loss, night sweats) for ≥2 weeks being initiated on anti-TB therapy.
Interventions: On enrolment, we obtained urine and nebulised sputum specimens, offered HIV testing and started participants on anti-TB therapy for ≥6 months. We collected urine samples after the 2-month intensive treatment phase and at the completion of anti-TB therapy. Positive LAM results were graded from 1 (low) to 5 (high). Participants were followed for >3 years.
Outcome measures: The primary outcome was change in urine LAM results during anti-TB therapy. The secondary outcome was all-cause mortality.
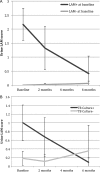
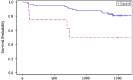
Results: Among 90 participants, 57 (63%) had culture-confirmed pulmonary TB. Among the 88 participants tested, 82 (93%) were HIV-infected with median CD4 168/mm(3) (IQR 89-256/mm(3)). During anti-TB therapy, the percentage of LAM-positive participants decreased from baseline to 2 months (32% to 16%), and from baseline to 6-months (32% to 10%) (p values <0.005). In multivariate longitudinal analyses, urine LAM positivity and grade decreased among those with culture-confirmed pulmonary TB (p<0.0001), and had no change in sputum culture-negative participants. At the 2-month visit, participants with positive laboratory-based LAM or rapid LAM with ≥2+ grade had a significantly greater risk of mortality. In analyses adjusted for age, sex, baseline Karnofsky score and HIV status, participants with a rapid LAM ≥2+ grade after 2 months of anti-TB therapy had a 5.6-fold (95% CI 1.2 to 25.2) greater risk of mortality.
Conclusions: Rapid urine LAM testing may be a valuable tool to monitor anti-TB therapy response and to assess prognosis of patients being treated for pulmonary TB in HIV-endemic regions.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures
References
-
- World Health Organization. Global TB Report 2012. Geneva: World Health Organization, 2012.
-
- World Health Organization. Joint TB/HIV Interventions. Geneva: World Health Organization, 2009.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
