A role for retinoids in human oocyte fertilization: regulation of connexin 43 by retinoic acid in cumulus granulosa cells
- PMID: 25877907
- PMCID: PMC4447995
- DOI: 10.1093/molehr/gav017
A role for retinoids in human oocyte fertilization: regulation of connexin 43 by retinoic acid in cumulus granulosa cells
Abstract
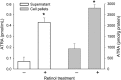
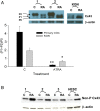
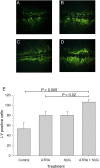
Retinoids are essential for ovarian steroid production and oocyte maturation in mammals. Oocyte competency is known to positively correlate with efficient gap junction intercellular communication (GJIC) among granulosa cells in the cumulus-oocyte complex. Connexin 43 (C x 43) is the main subunit of gap junction channels in human cumulus granulosa cells (CGC) and is regulated by all-trans retinoic acid (ATRA) in other hormone responsive cell types. The objectives of this study were to quantify retinoid levels in human CGC obtained during IVF oocyte retrievals, to investigate the potential relationship between CGC ATRA levels and successful oocyte fertilization, and to determine the effects of ATRA on C x 43 protein expression in CGC. Results showed that CGC cultures actively metabolize retinol to produce ATRA. Grouped according to fertilization rate tertiles, mean ATRA levels were 2-fold higher in pooled CGC from women in the highest versus the lowest tertile (P < 0.05). ATRA induced a rapid dephosphorylation of C x 43 in CGC and granulosa cell line (KGN) cultures resulting in a >2-fold increase in the expression of the functional non-phosphorylated (P0) species (P < 0.02). Similar enhancement of P0 by ATRA was shown in CGC and KGN cultures co-treated with LH or hCG which, by themselves, enhanced the protein levels of C x 43 without altering its phosphorylation profile. Correspondingly, the combination of ATRA+hCG treatment of KGN caused a significant increase in GJIC compared with single agent treatments (P < 0.025) and a doubling of GJIC from that seen in untreated cells (P < 0.01). These findings indicate that CGC are a primary site of retinoid uptake and ATRA biosynthesis. Regulation of C x 43 by ATRA may serve an important role in folliculogenesis, development of oocyte competency, and successful fertilization by increasing GJIC in CGC.
Keywords: all-trans retinoic acid; connexin 43; cumulus granulosa cells; oocyte competency.
© The Author 2015. Published by Oxford University Press on behalf of the European Society of Human Reproduction and Embryology. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures


References
-
- Ackert CL, Gittens JE, O'Brien MJ, Eppig JJ, Kidder GM. Intercellular communication via connexin43 gap junctions is required for ovarian folliculogenesis in the mouse. Dev Biol 2001;233:258–270. - PubMed
-
- Alminana C, Gil MA, Cuello C, Caballero I, Roca J, Vazquez JM, Gomez E, Martinez EA. In vitro maturation of porcine oocytes with retinoids improves embryonic development. Reprod Fertil Dev 2008;20:483–489. - PubMed
-
- Borowczyk E, Johnson ML, Bilski JJ, Bilska MA, Redmer DA, Reynolds LP, Grazul-Bilska AT. Role of gap junctions in regulation of progesterone secretion by ovine luteal cells in vitro. Reproduction 2007;133:641–651. - PubMed
-
- Brown JA, Eberhardt DM, Schrick FN, Roberts MP, Godkin JD. Expression of retinol-binding protein and cellular retinol-binding protein in the bovine ovary. Mol Reprod Dev 2003;64:261–269. - PubMed
-
- Dekel N. Cellular, biochemical and molecular mechanisms regulating oocyte maturation. Mol Cell Endocrinol 2005;234:19–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

