Recruitment and Screening for the Testosterone Trials
- PMID: 25878029
- PMCID: PMC4861649
- DOI: 10.1093/gerona/glv031
Recruitment and Screening for the Testosterone Trials
Abstract
Background: We describe the recruitment of men for The Testosterone (T) Trials, which were designed to determine the efficacy of T treatment.
Methods: Men were eligible if they were ≥65 years, had an average of two morning total T values <275 ng/dL with neither value >300 ng/mL, and had symptoms and objective evidence of mobility limitation, sexual dysfunction, and/or low vitality. Men had to be eligible for and enroll in at least one of these three main trials (physical function, sexual function, vitality).
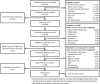
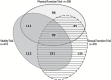
Results: Men were recruited primarily through mass mailings in 12 U.S. communities: 82% of men who contacted the sites did so in response to mailings. Men who responded were screened by telephone to ascertain eligibility. Of 51,085 telephone screens, 53.5% were eligible for further screening. Of 23,889 initial screening visits (SV1), 2,781 (11.6%) men were eligible for the second screening visit (SV2), which 2,261 (81.3%) completed. At SV2, 931 (41.2%) men met the criteria for one or more trials, the T level criterion and had no other exclusions. Of these, 790 (84.6%) were randomized; 99 (12.5%) in all three trials and 348 (44%) in two trials. Their mean age was 72 years and mean body mass index (BMI) was 31.0 kg/m(2). Mean (standard deviation) total T (ng/dL) was 212.0 (40.0).
Conclusion: Despite the telephone screening to enrollment ratio of 65 to 1, we met the recruitment goals for each trial. Recruitment of symptomatic older men with low testosterone levels is difficult but feasible.
Keywords: Hypogonadal men; Physical function; Randomized clinical trials.; Recruitment; Sexual function; Testosterone treatment; Vitality.
© The Author 2015. Published by Oxford University Press on behalf of The Gerontological Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Kasenda B, von Elm E, You J, et al. Prevalence, characteristics, and publication of discontinued randomized trials. JAMA. 2014;311:1045–1051. doi:10.1001/jama.2014.1361 - PubMed
-
- Weiss CO, Boyd CM, Yu Q, Wolff JL, Leff B. Patterns of prevalent major chronic disease among older adults in the United States. JAMA. 2007;298:1160–1162. doi:298/10/1160-a [pii]10.1001/jama.298. 10.1160-b - PubMed
-
- Cella D, Lai JS, Chang CH, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94:528–538. doi:10.1002/cncr.10245 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30-AG021342/AG/NIA NIH HHS/United States
- P30 AG024827/AG/NIA NIH HHS/United States
- U01 AG030644/AG/NIA NIH HHS/United States
- R01 AG037679/AG/NIA NIH HHS/United States
- P30 DK079626/DK/NIDDK NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- UL1 TR000142/TR/NCATS NIH HHS/United States
- U01 AG034661/AG/NIA NIH HHS/United States
- U01AG030644/AG/NIA NIH HHS/United States
- K24 AG021507/AG/NIA NIH HHS/United States
- DK079626/DK/NIDDK NIH HHS/United States
- P30 AG028740/AG/NIA NIH HHS/United States
- K24-AG021507/AG/NIA NIH HHS/United States
- P30 AG021342/AG/NIA NIH HHS/United States
- P60 DK079626/DK/NIDDK NIH HHS/United States
- UL1 TR001073/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

