EFTUD2 Is a Novel Innate Immune Regulator Restricting Hepatitis C Virus Infection through the RIG-I/MDA5 Pathway
- PMID: 25878102
- PMCID: PMC4468487
- DOI: 10.1128/JVI.00364-15
EFTUD2 Is a Novel Innate Immune Regulator Restricting Hepatitis C Virus Infection through the RIG-I/MDA5 Pathway
Abstract
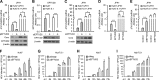
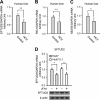
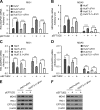
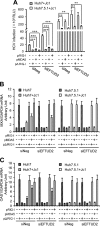
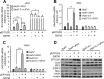
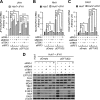
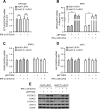
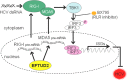
The elongation factor Tu GTP binding domain-containing protein 2 (EFTUD2) was identified as an anti-hepatitis C virus (HCV) host factor in our recent genome-wide small interfering RNA (siRNA) screen. In this study, we sought to further determine EFTUD2's role in HCV infection and investigate the interaction between EFTUD2 and other regulators involved in HCV innate immune (RIG-I, MDA5, TBK1, and IRF3) and JAK-STAT1 pathways. We found that HCV infection decreased the expression of EFTUD2 and the viral RNA sensors RIG-I and MDA5 in HCV-infected Huh7 and Huh7.5.1 cells and in liver tissue from in HCV-infected patients, suggesting that HCV infection downregulated EFTUD2 expression to circumvent the innate immune response. EFTUD2 inhibited HCV infection by inducing expression of the interferon (IFN)-stimulated genes (ISGs) in Huh7 cells. However, its impact on HCV infection was absent in both RIG-I knockdown Huh7 cells and RIG-I-defective Huh7.5.1 cells, indicating that the antiviral effect of EFTUD2 is dependent on RIG-I. Furthermore, EFTUD2 upregulated the expression of the RIG-I-like receptors (RLRs) RIG-I and MDA5 to enhance the innate immune response by gene splicing. Functional experiments revealed that EFTUD2-induced expression of ISGs was mediated through interaction of the EFTUD2 downstream regulators RIG-I, MDA5, TBK1, and IRF3. Interestingly, the EFTUD2-induced antiviral effect was independent of the classical IFN-induced JAK-STAT pathway. Our data demonstrate that EFTUD2 restricts HCV infection mainly through an RIG-I/MDA5-mediated, JAK-STAT-independent pathway, thereby revealing the participation of EFTUD2 as a novel innate immune regulator and suggesting a potentially targetable antiviral pathway.
Importance: Innate immunity is the first line defense against HCV and determines the outcome of HCV infection. Based on a recent high-throughput whole-genome siRNA library screen revealing a network of host factors mediating antiviral effects against HCV, we identified EFTUD2 as a novel innate immune regulator against HCV in the infectious HCV cell culture model and confirmed that its expression in HCV-infected liver tissue is inversely related to HCV infection. Furthermore, we determined that EFTUD2 exerts its antiviral activity mainly through governing its downstream regulators RIG-I and MDA5 by gene splicing to activate IRF3 and induce classical ISG expression independent of the JAT-STAT signaling pathway. This study broadens our understanding of the HCV innate immune response and provides a possible new antiviral strategy targeting this novel regulator of the innate response.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

Comment in
-
EFTUD2 on innate immunity.Oncotarget. 2015 Oct 20;6(32):32313-4. doi: 10.18632/oncotarget.5863. Oncotarget. 2015. PMID: 26427044 Free PMC article. No abstract available.
Similar articles
-
EFTUD2 on innate immunity.Oncotarget. 2015 Oct 20;6(32):32313-4. doi: 10.18632/oncotarget.5863. Oncotarget. 2015. PMID: 26427044 Free PMC article. No abstract available.
-
DDX60L Is an Interferon-Stimulated Gene Product Restricting Hepatitis C Virus Replication in Cell Culture.J Virol. 2015 Oct;89(20):10548-68. doi: 10.1128/JVI.01297-15. Epub 2015 Aug 12. J Virol. 2015. PMID: 26269178 Free PMC article.
-
Hepatitis C Virus Infection Is Inhibited by a Noncanonical Antiviral Signaling Pathway Targeted by NS3-NS4A.J Virol. 2019 Nov 13;93(23):e00725-19. doi: 10.1128/JVI.00725-19. Print 2019 Dec 1. J Virol. 2019. PMID: 31534039 Free PMC article.
-
Hepatitis A and hepatitis C viruses: divergent infection outcomes marked by similarities in induction and evasion of interferon responses.Semin Liver Dis. 2010 Nov;30(4):319-32. doi: 10.1055/s-0030-1267534. Epub 2010 Oct 19. Semin Liver Dis. 2010. PMID: 20960373 Review.
-
[RIG-I mediated hepatic innate immune signaling that controls HCV infection].Uirusu. 2008 Dec;58(2):105-15. doi: 10.2222/jsv.58.105. Uirusu. 2008. PMID: 19374189 Review. Japanese.
Cited by
-
HCV induces transforming growth factor β1 through activation of endoplasmic reticulum stress and the unfolded protein response.Sci Rep. 2016 Mar 1;6:22487. doi: 10.1038/srep22487. Sci Rep. 2016. PMID: 26927933 Free PMC article.
-
The Dengue Virus NS5 Protein Intrudes in the Cellular Spliceosome and Modulates Splicing.PLoS Pathog. 2016 Aug 30;12(8):e1005841. doi: 10.1371/journal.ppat.1005841. eCollection 2016 Aug. PLoS Pathog. 2016. PMID: 27575636 Free PMC article.
-
U5 snRNP Core Proteins Are Key Components of the Defense Response against Viral Infection through Their Roles in Programmed Cell Death and Interferon Induction.Viruses. 2022 Dec 3;14(12):2710. doi: 10.3390/v14122710. Viruses. 2022. PMID: 36560714 Free PMC article.
-
Comparative Transcriptomic Analysis of Immune-Related Gene Expression in Duck Embryo Fibroblasts Following Duck Tembusu Virus Infection.Int J Mol Sci. 2018 Aug 8;19(8):2328. doi: 10.3390/ijms19082328. Int J Mol Sci. 2018. PMID: 30096804 Free PMC article.
-
EFTUD2 is a promising diagnostic and prognostic indicator involved in the tumor immune microenvironment and glycolysis of lung adenocarcinoma.Front Oncol. 2025 Apr 1;15:1499217. doi: 10.3389/fonc.2025.1499217. eCollection 2025. Front Oncol. 2025. PMID: 40236649 Free PMC article.
References
-
- Sumpter R Jr, Loo YM, Foy E, Li K, Yoneyama M, Fujita T, Lemon SM, Gale M Jr. 2005. Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J Virol 79:2689–2699. doi:10.1128/JVI.79.5.2689-2699.2005. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

