Therapeutic immunization with a mixture of herpes simplex virus 1 glycoprotein D-derived “asymptomatic” human CD8+ T-cell epitopes decreases spontaneous ocular shedding in latently infected HLA transgenic rabbits: association with low frequency of local PD-1+ TIM-3+ CD8+ exhausted T cells
- PMID: 25878105
- PMCID: PMC4468472
- DOI: 10.1128/JVI.00788-15
Therapeutic immunization with a mixture of herpes simplex virus 1 glycoprotein D-derived “asymptomatic” human CD8+ T-cell epitopes decreases spontaneous ocular shedding in latently infected HLA transgenic rabbits: association with low frequency of local PD-1+ TIM-3+ CD8+ exhausted T cells
Abstract
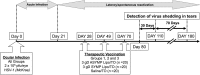
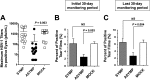
Most blinding ocular herpetic disease is due to reactivation of herpes simplex virus 1 (HSV-1) from latency rather than to primary acute infection. No herpes simplex vaccine is currently available for use in humans. In this study, we used the HLA-A*02:01 transgenic (HLA Tg) rabbit model of ocular herpes to assess the efficacy of a therapeutic vaccine based on HSV-1 gD epitopes that are recognized mainly by CD8(+) T cells from "naturally" protected HLA-A*02:01-positive, HSV-1-seropositive healthy asymptomatic (ASYMP) individuals (who have never had clinical herpes disease). Three ASYMP CD8(+) T-cell epitopes (gD(53-61), gD(70-78), and gD(278-286)) were linked with a promiscuous CD4(+) T-cell epitope (gD(287-317)) to create 3 separate pairs of CD4-CD8 peptides, which were then each covalently coupled to an Nε-palmitoyl-lysine moiety, a Toll-like receptor 2 (TLR-2) ligand. This resulted in the construction of 3 CD4-CD8 lipopeptide vaccines. Latently infected HLA Tg rabbits were immunized with a mixture of these 3 ASYMP lipopeptide vaccines, delivered as eye drops in sterile phosphate-buffered saline (PBS). The ASYMP therapeutic vaccination (i) induced HSV-specific CD8(+) T cells that prevent HSV-1 reactivation ex vivo from latently infected explanted trigeminal ganglia (TG), (ii) significantly reduced HSV-1 shedding detected in tears, (iii) boosted the number and function of HSV-1 gD epitope-specific CD8(+) T cells in draining lymph nodes (DLN), conjunctiva, and TG, and (iv) was associated with fewer exhausted HSV-1 gD-specific PD-1(+) TIM-3+ CD8(+) T cells. The results underscore the potential of an ASYMP CD8(+) T-cell epitope-based therapeutic vaccine strategy against recurrent ocular herpes.
Importance: Seventy percent to 90% of adults harbor herpes simplex virus 1 (HSV-1), which establishes lifelong latency in sensory neurons of the trigeminal ganglia. This latent state sporadically switches to spontaneous reactivation, resulting in viral shedding in tears. Most blinding herpetic disease in humans is due to reactivation of HSV-1 from latency rather than to primary acute infection. To date, there is no licensed therapeutic vaccine that can effectively stop or reduce HSV-1 reactivation from latently infected sensory ganglia and the subsequent shedding in tears. In the present study, we demonstrated that topical ocular therapeutic vaccination of latently infected HLA transgenic rabbits with a lipopeptide vaccine that contains exclusively human “asymptomatic” CD8(+) T-cell epitopes successfully decreased spontaneous HSV-1 reactivation, as judged by a significant reduction in spontaneous shedding in tears. The findings should guide the clinical development of a safe and effective T-cell-based therapeutic herpes vaccine.
Figures





Similar articles
-
A novel HLA (HLA-A*0201) transgenic rabbit model for preclinical evaluation of human CD8+ T cell epitope-based vaccines against ocular herpes.J Immunol. 2010 Mar 1;184(5):2561-71. doi: 10.4049/jimmunol.0902322. Epub 2010 Feb 1. J Immunol. 2010. PMID: 20124097 Free PMC article.
-
A tissue-targeted prime/pull/keep therapeutic herpes simplex virus vaccine protects against recurrent ocular herpes infection and disease in HLA-A*0201 transgenic rabbits.J Virol. 2025 May 20;99(5):e0013525. doi: 10.1128/jvi.00135-25. Epub 2025 Apr 10. J Virol. 2025. PMID: 40207928 Free PMC article.
-
Human Asymptomatic Epitope Peptide/CXCL10-Based Prime/Pull Vaccine Induces Herpes Simplex Virus-Specific Gamma Interferon-Positive CD107+ CD8+ T Cells That Infiltrate the Corneas and Trigeminal Ganglia of Humanized HLA Transgenic Rabbits and Protect against Ocular Herpes Challenge.J Virol. 2018 Jul 31;92(16):e00535-18. doi: 10.1128/JVI.00535-18. Print 2018 Aug 15. J Virol. 2018. PMID: 29899087 Free PMC article.
-
Of mice and not humans: how reliable are animal models for evaluation of herpes CD8(+)-T cell-epitopes-based immunotherapeutic vaccine candidates?Vaccine. 2011 Aug 11;29(35):5824-36. doi: 10.1016/j.vaccine.2011.06.083. Epub 2011 Jun 28. Vaccine. 2011. PMID: 21718746 Free PMC article. Review.
-
Towards a rational design of an asymptomatic clinical herpes vaccine: the old, the new, and the unknown.Clin Dev Immunol. 2012;2012:187585. doi: 10.1155/2012/187585. Epub 2012 Mar 26. Clin Dev Immunol. 2012. PMID: 22548113 Free PMC article. Review.
Cited by
-
Human Epitopes Identified from Herpes Simplex Virus Tegument Protein VP11/12 (UL46) Recall Multifunctional Effector Memory CD4+ TEM Cells in Asymptomatic Individuals and Protect from Ocular Herpes Infection and Disease in "Humanized" HLA-DR Transgenic Mice.J Virol. 2020 Mar 17;94(7):e01991-19. doi: 10.1128/JVI.01991-19. Print 2020 Mar 17. J Virol. 2020. PMID: 31915285 Free PMC article.
-
Prior Corneal Scarification and Injection of Immune Serum are Not Required Before Ocular HSV-1 Infection for UV-B-Induced Virus Reactivation and Recurrent Herpetic Corneal Disease in Latently Infected Mice.Curr Eye Res. 2016 Jun;41(6):747-56. doi: 10.3109/02713683.2015.1061024. Epub 2015 Sep 23. Curr Eye Res. 2016. PMID: 26398722 Free PMC article.
-
Local Immune Control of Latent Herpes Simplex Virus Type 1 in Ganglia of Mice and Man.Front Immunol. 2021 Sep 15;12:723809. doi: 10.3389/fimmu.2021.723809. eCollection 2021. Front Immunol. 2021. PMID: 34603296 Free PMC article. Review.
-
Bolstering the Number and Function of HSV-1-Specific CD8+ Effector Memory T Cells and Tissue-Resident Memory T Cells in Latently Infected Trigeminal Ganglia Reduces Recurrent Ocular Herpes Infection and Disease.J Immunol. 2017 Jul 1;199(1):186-203. doi: 10.4049/jimmunol.1700145. Epub 2017 May 24. J Immunol. 2017. PMID: 28539429 Free PMC article.
-
The Herpes Simplex Virus Latency-Associated Transcript Gene Is Associated with a Broader Repertoire of Virus-Specific Exhausted CD8+ T Cells Retained within the Trigeminal Ganglia of Latently Infected HLA Transgenic Rabbits.J Virol. 2016 Mar 28;90(8):3913-3928. doi: 10.1128/JVI.02450-15. Print 2016 Apr. J Virol. 2016. PMID: 26842468 Free PMC article.
References
-
- Samandary S, Kridane-Miledi H, Sandoval JS, Choudhury Z, Langa-Vives F, Spencer D, Chentoufi AA, Lemonnier FA, BenMohamed L. 2014. Associations of HLA-A, HLA-B and HLA-C alleles frequency with prevalence of herpes simplex virus infections and diseases across global populations: implication for the development of an universal CD8+ T-cell epitope-based vaccine. Hum Immunol 75:715–729. doi:10.1016/j.humimm.2014.04.016. - DOI - PMC - PubMed
-
- Dervillez X, Qureshi H, Chentoufi AA, Khan AA, Kritzer E, Yu DC, Diaz OR, Gottimukkala C, Kalantari M, Villacres MC, Scarfone VM, McKinney DM, Sidney J, Sette A, Nesburn AB, Wechsler SL, BenMohamed L. 2013. Asymptomatic HLA-A*02:01-restricted epitopes from herpes simplex virus glycoprotein B preferentially recall polyfunctional CD8+ T cells from seropositive asymptomatic individuals and protect HLA transgenic mice against ocular herpes. J Immunol 191:5124–5138. doi:10.4049/jimmunol.1301415. - DOI - PMC - PubMed
-
- Chentoufi AA, Zhang X, Lamberth K, Dasgupta G, Bettahi I, Nguyen A, Wu M, Zhu X, Mohebbi A, Buus S, Wechsler SL, Nesburn AB, BenMohamed L. 2008. HLA-A*0201-restricted CD8+ cytotoxic T lymphocyte epitopes identified from herpes simplex virus glycoprotein D. J Immunol 180:426–437. doi:10.4049/jimmunol.180.1.426. - DOI - PubMed
-
- Zhang X, Dervillez X, Chentoufi AA, Badakhshan T, Bettahi I, Benmohamed L. 2012. Targeting the genital tract mucosa with a lipopeptide/recombinant adenovirus prime/boost vaccine induces potent and long-lasting CD8+ T cell immunity against herpes: importance of MyD88. J Immunol 189:4496–4509. doi:10.4049/jimmunol.1201121. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

