Magnetic resonance imaging-based computational modelling of blood flow and nanomedicine deposition in patients with peripheral arterial disease
- PMID: 25878124
- PMCID: PMC4424675
- DOI: 10.1098/rsif.2015.0001
Magnetic resonance imaging-based computational modelling of blood flow and nanomedicine deposition in patients with peripheral arterial disease
Abstract
Peripheral arterial disease (PAD) is generally attributed to the progressive vascular accumulation of lipoproteins and circulating monocytes in the vessel walls leading to the formation of atherosclerotic plaques. This is known to be regulated by the local vascular geometry, haemodynamics and biophysical conditions. Here, an isogeometric analysis framework is proposed to analyse the blood flow and vascular deposition of circulating nanoparticles (NPs) into the superficial femoral artery (SFA) of a PAD patient. The local geometry of the blood vessel and the haemodynamic conditions are derived from magnetic resonance imaging (MRI), performed at baseline and at 24 months post intervention. A dramatic improvement in blood flow dynamics is observed post intervention. A 500% increase in peak flow rate is measured in vivo as a consequence of luminal enlargement. Furthermore, blood flow simulations reveal a 32% drop in the mean oscillatory shear index, indicating reduced disturbed flow post intervention. The same patient information (vascular geometry and blood flow) is used to predict in silico in a simulation of the vascular deposition of systemically injected nanomedicines. NPs, targeted to inflammatory vascular molecules including VCAM-1, E-selectin and ICAM-1, are predicted to preferentially accumulate near the stenosis in the baseline configuration, with VCAM-1 providing the highest accumulation (approx. 1.33 and 1.50 times higher concentration than that of ICAM-1 and E-selectin, respectively). Such selective deposition of NPs within the stenosis could be effectively used for the detection and treatment of plaques forming in the SFA. The presented MRI-based computational protocol can be used to analyse data from clinical trials to explore possible correlations between haemodynamics and disease progression in PAD patients, and potentially predict disease occurrence as well as the outcome of an intervention.
Keywords: atherosclerosis; finite-element modelling; magnetic resonance imaging; nanoparticles; vascular adhesion.
© 2015 The Author(s) Published by the Royal Society. All rights reserved.
Figures



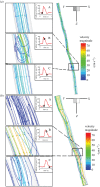



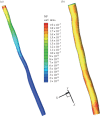
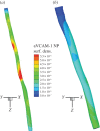


 . Addition of TNF-α under static conditions stimulated upregulation of VCAM-1 by 350%, ICAM-1 by 150% and E-selectin by 250% [44]. (Online version in colour.)
. Addition of TNF-α under static conditions stimulated upregulation of VCAM-1 by 350%, ICAM-1 by 150% and E-selectin by 250% [44]. (Online version in colour.)References
-
- Regensteiner JG, Hiatt WR, Coll JR, Criqui MH, Treat-Jacobson D, McDermott MM, Hirsch AT. 2008. The impact of peripheral arterial disease on health-related quality of life in the peripheral arterial disease awareness, risk, and treatment: new resources for survival (PARTNERS) program. Vasc. Med. 13, 15–24. ( 10.1177/1358863X07084911) - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
