Intrahippocampal glutamine administration inhibits mTORC1 signaling and impairs long-term memory
- PMID: 25878136
- PMCID: PMC4408772
- DOI: 10.1101/lm.038265.115
Intrahippocampal glutamine administration inhibits mTORC1 signaling and impairs long-term memory
Abstract
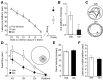
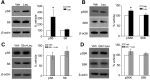
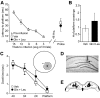
The mechanistic Target of Rapamycin Complex 1 (mTORC1), a key regulator of protein synthesis and cellular growth, is also required for long-term memory formation. Stimulation of mTORC1 signaling is known to be dependent on the availability of energy and growth factors, as well as the presence of amino acids. In vitro studies using serum- and amino acid-starved cells have reported that glutamine addition can either stimulate or repress mTORC1 activity, depending on the particular experimental system that was used. However, these experiments do not directly address the effect of glutamine on mTORC1 activity under physiological conditions in nondeprived cells in vivo. We present experimental results indicating that intrahippocampal administration of glutamine to rats reduces mTORC1 activity. Moreover, post-training administration of glutamine impairs long-term spatial memory formation, while coadministration of glutamine with leucine had no influence on memory. Intracellular recordings in hippocampal slices showed that glutamine did not alter either excitatory or inhibitory synaptic activity, suggesting that the observed memory impairments may not result from conversion of glutamine to either glutamate or GABA. Taken together, these findings indicate that glutamine can decrease mTORC1 activity in the brain and may have implications for treatments of neurological diseases associated with high mTORC1 signaling.
© 2015 Rozas et al.; Published by Cold Spring Harbor Laboratory Press.
Figures
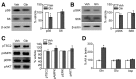
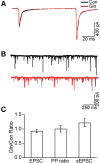
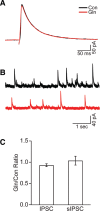
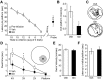
References
-
- Albrecht J, Sidoryk-Wegrzynowicz M, Zielińska M, Aschner M 2010. Roles of glutamine in neurotransmission. Neuron Glia Biol 6: 263–276. - PubMed
-
- Averous J, Lambert-Langlais S, Carraro V, Gourbeyre O, Parry L, B'Chir W, Muranishi Y, Jousse C, Bruhat A, Maurin AC, et al. 2014. Requirement for lysosomal localization of mTOR for its activation differs between leucine and other amino acids. Cell Signal 26: 1918–1927. - PubMed
-
- Barondes SH, Cohen HD 1968. Memory impairment after subcutaneous injection of acetoxycycloheximide. Science 160: 556–557. - PubMed
-
- Blum S, Dash PK 2004. A cell-permeable phospholipase Cγ1-binding peptide transduces neurons and impairs long-term spatial memory. Learn Mem 11: 239–243. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
