Oxytocin signaling in basolateral and central amygdala nuclei differentially regulates the acquisition, expression, and extinction of context-conditioned fear in rats
- PMID: 25878137
- PMCID: PMC4408769
- DOI: 10.1101/lm.036962.114
Oxytocin signaling in basolateral and central amygdala nuclei differentially regulates the acquisition, expression, and extinction of context-conditioned fear in rats
Abstract
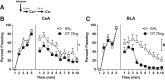
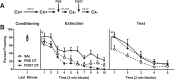
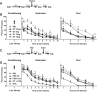
The present study investigated how oxytocin (OT) signaling in the central (CeA) and basolateral (BLA) amygdala affects acquisition, expression, and extinction of context-conditioned fear (freezing) in rats. In the first set of experiments, acquisition of fear to a shocked context was impaired by a preconditioning infusion of synthetic OT into the CeA (Experiment 1) or BLA (Experiment 2). In the second set of experiments, expression of context fear was enhanced by a pre- or post-extinction CeA infusion of synthetic OT (Experiments 3-6) or a selective OT receptor agonist, TGOT (Experiment 4). This enhancement of fear was blocked by coadministration of an OT receptor antagonist, OTA (Experiment 5) and context fear was suppressed by administration of the antagonist alone (Experiment 6). In the third set of experiments, expression of context fear was suppressed, not enhanced, by a preextinction BLA infusion of synthetic OT or a selective OT receptor agonist, TGOT (Experiments 7 and 8). This suppression of fear was blocked by coadministration of the OT receptor antagonist, OTA (Experiment 8). Taken together, these findings show that the involvement of the CeA and BLA in expression and extinction of context-conditioned fear is dissociable, and imply a critical role for oxytocin signaling in amygdala-based regulation of aversive learning.
© 2015 Campbell-Smith et al.; Published by Cold Spring Harbor Laboratory Press.
Figures


Similar articles
-
NMDA receptor antagonism in the basolateral but not central amygdala blocks the extinction of Pavlovian fear conditioning in rats.Eur J Neurosci. 2010 May;31(9):1664-70. doi: 10.1111/j.1460-9568.2010.07223.x. Eur J Neurosci. 2010. PMID: 20525079 Free PMC article.
-
Role of the basolateral amygdala in the reinstatement and extinction of fear responses to a previously extinguished conditioned stimulus.Learn Mem. 2010 Feb 13;17(2):86-96. doi: 10.1101/lm.1655010. Print 2010 Feb. Learn Mem. 2010. PMID: 20154354
-
Oxytocin in the amygdala and not the prefrontal cortex enhances fear and impairs extinction in the juvenile rat.Neurobiol Learn Mem. 2017 May;141:179-188. doi: 10.1016/j.nlm.2017.04.001. Epub 2017 Apr 5. Neurobiol Learn Mem. 2017. PMID: 28389281
-
The role of the amygdala in the extinction of conditioned fear.Biol Psychiatry. 2006 Aug 15;60(4):322-8. doi: 10.1016/j.biopsych.2006.05.029. Biol Psychiatry. 2006. PMID: 16919522 Review.
-
The role of the basolateral amygdala and infralimbic cortex in (re)learning extinction.Psychopharmacology (Berl). 2019 Jan;236(1):303-312. doi: 10.1007/s00213-018-4957-x. Epub 2018 Jun 30. Psychopharmacology (Berl). 2019. PMID: 29959461 Review.
Cited by
-
Oxytocin receptor activation does not mediate associative fear deficits in a Williams Syndrome model.Genes Brain Behav. 2022 Jan;21(1):e12750. doi: 10.1111/gbb.12750. Epub 2021 Jun 10. Genes Brain Behav. 2022. PMID: 33978321 Free PMC article.
-
Oxytocin and Fear Memory Extinction: Possible Implications for the Therapy of Fear Disorders?Int J Mol Sci. 2021 Sep 16;22(18):10000. doi: 10.3390/ijms221810000. Int J Mol Sci. 2021. PMID: 34576161 Free PMC article. Review.
-
Effects of route of administration on oxytocin-induced changes in regional cerebral blood flow in humans.Nat Commun. 2020 Mar 3;11(1):1160. doi: 10.1038/s41467-020-14845-5. Nat Commun. 2020. PMID: 32127545 Free PMC article. Clinical Trial.
-
Oxytocin activity in the paraventricular and supramammillary nuclei of the hypothalamus is essential for social recognition memory in rats.Mol Psychiatry. 2024 Feb;29(2):412-424. doi: 10.1038/s41380-023-02336-0. Epub 2023 Dec 5. Mol Psychiatry. 2024. PMID: 38052983 Free PMC article.
-
Acute and long-lasting effects of oxytocin in cortico-limbic circuits: consequences for fear recall and extinction.Psychopharmacology (Berl). 2019 Jan;236(1):339-354. doi: 10.1007/s00213-018-5030-5. Epub 2018 Oct 9. Psychopharmacology (Berl). 2019. PMID: 30302511 Review.
References
-
- Bartz JA, Zaki J, Bolger N, Ochsner KN 2011. Social effects of oxytocin in humans: context and person matter. Trends Cogn Sci 15: 301–309. - PubMed
-
- Betz MA, Gabriel KR 1978. Type IV errors and analysis of simple effects. J Educ Stat 3: 121–143.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
