NMDA receptors are the basis for persistent network activity in neocortex slices
- PMID: 25878152
- PMCID: PMC4473514
- DOI: 10.1152/jn.00090.2015
NMDA receptors are the basis for persistent network activity in neocortex slices
Abstract
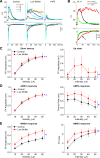
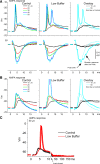
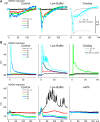
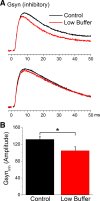
During behavioral quiescence the neocortex generates spontaneous slow oscillations that consist of Up and Down states. Up states are short epochs of persistent activity, but their underlying source is unclear. In neocortex slices of adult mice, we monitored several cellular and network variables during the transition between a traditional buffer, which does not cause Up states, and a lower-divalent cation buffer, which leads to the generation of Up states. We found that the resting membrane potential and input resistance of cortical cells did not change with the development of Up states. The synaptic efficacy of excitatory postsynaptic potentials mediated by non-NMDA receptors was slightly reduced, but this is unlikely to facilitate the generation of Up states. On the other hand, we identified two variables that are associated with the generation of Up states: an enhancement of the intrinsic firing excitability of cortical cells and an enhancement of NMDA-mediated responses evoked by electrical or optogenetic stimulation. The fact that blocking NMDA receptors abolishes Up states indicates that the enhancement in intrinsic firing excitability alone is insufficient to generate Up states. NMDA receptors have a crucial role in the generation of Up states in neocortex slices.
Keywords: Down state; Up state; arousal; cortex; slice; slow oscillation.
Copyright © 2015 the American Physiological Society.
Figures


Similar articles
-
Homeostatic presynaptic suppression of neuronal network bursts.J Neurophysiol. 2009 Apr;101(4):2077-88. doi: 10.1152/jn.91085.2008. Epub 2009 Feb 4. J Neurophysiol. 2009. PMID: 19193770
-
Synaptic cooperativity regulates persistent network activity in neocortex.J Neurosci. 2013 Feb 13;33(7):3151-63. doi: 10.1523/JNEUROSCI.4424-12.2013. J Neurosci. 2013. PMID: 23407969 Free PMC article.
-
Non-fibrillar beta-amyloid abates spike-timing-dependent synaptic potentiation at excitatory synapses in layer 2/3 of the neocortex by targeting postsynaptic AMPA receptors.Eur J Neurosci. 2006 Apr;23(8):2035-47. doi: 10.1111/j.1460-9568.2006.04733.x. Eur J Neurosci. 2006. PMID: 16630051
-
Early NMDA receptor-driven waves of activity in the developing neocortex: physiological or pathological network oscillations?J Physiol. 2010 Jan 1;588(Pt 1):83-91. doi: 10.1113/jphysiol.2009.178798. Epub 2009 Nov 16. J Physiol. 2010. PMID: 19917570 Free PMC article. Review.
-
Cortical up and activated states: implications for sensory information processing.Neuroscientist. 2009 Dec;15(6):625-34. doi: 10.1177/1073858409333074. Neuroscientist. 2009. PMID: 19321459 Free PMC article. Review.
Cited by
-
The amyloid precursor family of proteins in excitatory neurons are essential for regulating cortico-hippocampal circuit dynamics in vivo.Cell Rep. 2025 Jun 24;44(6):115801. doi: 10.1016/j.celrep.2025.115801. Epub 2025 Jun 11. Cell Rep. 2025. PMID: 40512617 Free PMC article.
-
The Slow Oscillation in Cortical and Thalamic Networks: Mechanisms and Functions.Front Neural Circuits. 2016 Jan 14;9:88. doi: 10.3389/fncir.2015.00088. eCollection 2015. Front Neural Circuits. 2016. PMID: 26834569 Free PMC article. Review.
-
Effect of Neonatal Treatment With the NMDA Receptor Antagonist, MK-801, During Different Temporal Windows of Postnatal Period in Adult Prefrontal Cortical and Hippocampal Function.Front Behav Neurosci. 2021 Jun 11;15:689193. doi: 10.3389/fnbeh.2021.689193. eCollection 2021. Front Behav Neurosci. 2021. PMID: 34177484 Free PMC article.
-
Computational Account of Spontaneous Activity as a Signature of Predictive Coding.PLoS Comput Biol. 2017 Jan 23;13(1):e1005355. doi: 10.1371/journal.pcbi.1005355. eCollection 2017 Jan. PLoS Comput Biol. 2017. PMID: 28114353 Free PMC article.
-
Distinct mechanisms of Up state maintenance in the medial entorhinal cortex and neocortex.Neuropharmacology. 2017 Feb;113(Pt A):543-555. doi: 10.1016/j.neuropharm.2016.11.009. Epub 2016 Nov 10. Neuropharmacology. 2017. PMID: 27838344 Free PMC article.
References
-
- Agmon A, Connors BW. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience 41: 365–379, 1991. - PubMed
-
- Berretta N, Jones RS. Tonic facilitation of glutamate release by presynaptic N-methyl-d-aspartate autoreceptors in the entorhinal cortex. Neuroscience 75: 339–344, 1996. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

