Time to prerandomization monthly seizure count in perampanel trials: A novel epilepsy endpoint
- PMID: 25878175
- PMCID: PMC4442101
- DOI: 10.1212/WNL.0000000000001585
Time to prerandomization monthly seizure count in perampanel trials: A novel epilepsy endpoint
Abstract
Objective: To determine whether a novel endpoint of time to prerandomization monthly seizure count could be used to differentiate efficacious and nonefficacious therapies in clinical trials of new add-on antiepileptic drugs (AEDs).
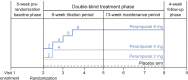
Methods: This analysis used data from 3 randomized, double-blind, placebo-controlled phase III trials of perampanel as an add-on therapy in patients with epilepsy who were experiencing refractory partial seizures: studies 304 (ClinicalTrials.gov identifier NCT00699972), 305 (NCT00699582), and 306 (NCT00700310). Time to prerandomization monthly seizure count was evaluated post hoc for each trial, and findings were compared with the original primary outcomes (median percent change in seizure frequency and 50% responder rate). Outcomes were assessed for all partial-onset seizures, secondarily generalized (SG) tonic-clonic seizures only, and complex partial plus SG (CP + SG) seizures.
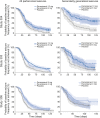
Results: Perampanel 4-12 mg significantly prolonged median time to prerandomization monthly seizure count, generally by more than 1 week, compared with placebo, across all 3 studies, consistent with the original primary outcomes. Analysis of SG seizures only, and CP + SG seizures, also indicated a significantly prolonged median time to prerandomization monthly seizure count with perampanel 8 mg and 12 mg compared with placebo.
Conclusions: Time to prerandomization monthly seizure count is a promising novel alternative to the standard endpoints of median percent change in seizure frequency and 50% responder rates used in trials of add-on AEDs. Use of this endpoint could reduce exposure to placebo or ineffective treatments, thereby facilitating trial recruitment and improving safety.
© 2015 American Academy of Neurology.
Figures
Comment in
-
Antiepileptic drug trials: Less placebo exposure for safer studies?Neurology. 2015 May 19;84(20):2010-1. doi: 10.1212/WNL.0000000000001597. Epub 2015 Apr 15. Neurology. 2015. PMID: 25878184 No abstract available.
Similar articles
-
Efficacy and safety of adjunctive perampanel in adolescent patients with epilepsy: Post hoc analysis of six randomized studies.Epilepsy Behav. 2020 Mar;104(Pt A):106876. doi: 10.1016/j.yebeh.2019.106876. Epub 2020 Jan 16. Epilepsy Behav. 2020. PMID: 31954998 Clinical Trial.
-
Efficacy and safety of adjunctive perampanel for the treatment of refractory partial seizures: a pooled analysis of three phase III studies.Epilepsia. 2013 Aug;54(8):1481-9. doi: 10.1111/epi.12212. Epub 2013 May 10. Epilepsia. 2013. PMID: 23663001 Clinical Trial.
-
Evaluation of adjunctive perampanel in patients with refractory partial-onset seizures: results of randomized global phase III study 305.Epilepsia. 2013 Jan;54(1):117-25. doi: 10.1111/j.1528-1167.2012.03638.x. Epub 2012 Aug 20. Epilepsia. 2013. PMID: 22905857 Clinical Trial.
-
Perampanel: expanding therapeutic options for patients with medically refractory secondary generalized convulsive seizures.Acta Neurol Scand Suppl. 2013;(197):36-43. doi: 10.1111/ane.12103. Acta Neurol Scand Suppl. 2013. PMID: 23480155 Review.
-
Perampanel as monotherapy and adjunctive therapy for focal onset seizures, focal to bilateral tonic-clonic seizures and as adjunctive therapy of generalized onset tonic-clonic seizures.Expert Rev Neurother. 2019 Jan;19(1):5-16. doi: 10.1080/14737175.2019.1555474. Epub 2018 Dec 18. Expert Rev Neurother. 2019. PMID: 30560703 Review.
Cited by
-
Time to exceed pre-randomization monthly seizure count for perampanel in participants with primary generalized tonic-clonic seizures: A potential clinical end point.Epilepsia. 2022 Nov;63(11):2994-3004. doi: 10.1111/epi.17411. Epub 2022 Sep 30. Epilepsia. 2022. PMID: 36106379 Free PMC article. Clinical Trial.
-
The present and future of seizure detection, prediction, and forecasting with machine learning, including the future impact on clinical trials.Front Neurol. 2024 Jul 11;15:1425490. doi: 10.3389/fneur.2024.1425490. eCollection 2024. Front Neurol. 2024. PMID: 39055320 Free PMC article. Review.
-
Time-to-event clinical trial designs: Existing evidence and remaining concerns.Epilepsia. 2023 Jul;64(7):1699-1708. doi: 10.1111/epi.17621. Epub 2023 May 2. Epilepsia. 2023. PMID: 37073881 Free PMC article. Review.
-
Analyses of seizure responses supportive of a novel trial design to assess efficacy of antiepileptic drugs in infants and young children with epilepsy: Post hoc analyses of pediatric levetiracetam and lacosamide trials.Epilepsia Open. 2021 Jun;6(2):359-368. doi: 10.1002/epi4.12482. Epub 2021 May 3. Epilepsia Open. 2021. PMID: 34033237 Free PMC article. Clinical Trial.
-
From clinical trials of antiepileptic drugs to treatment.Epilepsia Open. 2018 Jul 10;3(Suppl Suppl 2):220-230. doi: 10.1002/epi4.12239. eCollection 2018 Dec. Epilepsia Open. 2018. PMID: 30564781 Free PMC article.
References
-
- Ben-Menachem E, Falter U. Efficacy and tolerability of levetiracetam 3000 mg/d in patients with refractory partial seizures: a multicenter, double-blind, responder-selected study evaluating monotherapy: European Levetiracetam Study Group. Epilepsia 2000;41:1276–1283. - PubMed
-
- Ben-Menachem E, Biton V, Jatuzis D, Abou-Khalil B, Doty P, Rudd GD. Efficacy and safety of oral lacosamide as adjunctive therapy in adults with partial-onset seizures. Epilepsia 2007;48:1308–1317. - PubMed
-
- Ben-Menachem E, Gabbai AA, Hufnagel A, Maia J, Almeida L, Soares-da-Silva P. Eslicarbazepine acetate as adjunctive therapy in adult patients with partial epilepsy. Epilepsy Res 2010;89:278–285. - PubMed
-
- Brodie MJ, Duncan R, Vespignani H, Solyom A, Bitenskyy V, Lucas C. Dose-dependent safety and efficacy of zonisamide: a randomized, double-blind, placebo-controlled study in patients with refractory partial seizures. Epilepsia 2005;46:31–41. - PubMed
-
- Brodie MJ, Lerche H, Gil-Nagel A, et al. Efficacy and safety of adjunctive ezogabine (retigabine) in refractory partial epilepsy. Neurology 2010;75:1817–1824. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
