Perampanel efficacy and tolerability with enzyme-inducing AEDs in patients with epilepsy
- PMID: 25878177
- PMCID: PMC4433458
- DOI: 10.1212/WNL.0000000000001558
Perampanel efficacy and tolerability with enzyme-inducing AEDs in patients with epilepsy
Abstract
Objective: Evaluate the impact of concomitant enzyme (CYP3A4)-inducer antiepileptic drugs (EIAEDs) on the efficacy and safety of perampanel in patients from the 3 phase-III clinical trials.
Methods: Patients with pharmacoresistant partial-onset seizures in the 3 phase-III clinical studies were aged 12 years and older and receiving 1 to 3 concomitant antiepileptic drugs. Following 6-week baseline, patients were randomized to once-daily, double-blind treatment with placebo or perampanel 8 or 12 mg (studies 304 and 305) or placebo or perampanel 2, 4, or 8 mg (study 306).
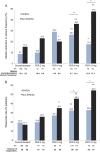
Results: Treatment response assessed by median percent reduction in seizure frequency and responder rates improved with perampanel compared with placebo. However, at 8 and 12 mg, the treatment response was significantly greater in patients receiving non-EIAEDs. The treatment effect (perampanel-placebo) also demonstrated a dose-dependent increase in all patients. The overall incidence of treatment-emergent adverse events was similar regardless of the presence of EIAEDs. Occurrence of some adverse events, such as fatigue, somnolence, dizziness, irritability, was greater in patients receiving non-EIAEDs, as was discontinuation because of adverse events.
Conclusions: Perampanel shows efficacy and safety in the presence and absence of EIAEDs. As systemic exposure to perampanel increases, so does efficacy. Given the extensive metabolism of perampanel, systemic exposure is clearly reduced with concomitant administration of CYP3A4 inducers. This supports the strategy of dosing perampanel to clinical effect. Recognition of these pharmacokinetic interactions will be important in the optimization of this novel medication.
Classification of evidence: This study provides Class II evidence that 2 to 12 mg/d doses of perampanel reduced seizure frequency and improved responder rate in the presence and absence of EIAEDs.
© 2015 American Academy of Neurology.
Figures
Similar articles
-
Concentration-effect relationships with perampanel in patients with pharmacoresistant partial-onset seizures.Epilepsia. 2013 Aug;54(8):1490-7. doi: 10.1111/epi.12240. Epub 2013 Jun 17. Epilepsia. 2013. PMID: 23772853 Clinical Trial.
-
Efficacy and tolerability of adjunct perampanel based on number of antiepileptic drugs at baseline and baseline predictors of efficacy: A phase III post-hoc analysis.Epilepsy Res. 2016 Jan;119:34-40. doi: 10.1016/j.eplepsyres.2015.11.014. Epub 2015 Dec 1. Epilepsy Res. 2016. PMID: 26656783 Clinical Trial.
-
Analysis of pooled phase III trials of adjunctive perampanel for epilepsy: Impact of mechanism of action and pharmacokinetics on clinical outcomes.Epilepsy Res. 2015 Nov;117:117-24. doi: 10.1016/j.eplepsyres.2015.09.002. Epub 2015 Sep 9. Epilepsy Res. 2015. PMID: 26448264 Clinical Trial.
-
Adverse effects and safety profile of perampanel: a review of pooled data.Epilepsia. 2014 Jan;55 Suppl 1:13-5. doi: 10.1111/epi.12504. Epilepsia. 2014. PMID: 24400692 Review.
-
Novel treatment options for epilepsy: focus on perampanel.Pharmacol Res. 2013 Apr;70(1):35-40. doi: 10.1016/j.phrs.2012.12.006. Epub 2013 Jan 1. Pharmacol Res. 2013. PMID: 23287426 Review.
Cited by
-
Preliminary Asian experience of using perampanel in clinical practice.Biomed J. 2017 Dec;40(6):347-354. doi: 10.1016/j.bj.2017.09.003. Epub 2018 Feb 3. Biomed J. 2017. PMID: 29433838 Free PMC article.
-
Perampanel: A Review in Drug-Resistant Epilepsy.Drugs. 2015 Sep;75(14):1657-68. doi: 10.1007/s40265-015-0465-z. Drugs. 2015. PMID: 26370209 Review.
-
Population Pharmacokinetic Analysis of Perampanel in Portuguese Patients Diagnosed with Refractory Epilepsy.Pharmaceutics. 2023 Jun 10;15(6):1704. doi: 10.3390/pharmaceutics15061704. Pharmaceutics. 2023. PMID: 37376153 Free PMC article.
-
Cytochrome P450-mediated estrogen catabolism therapeutic avenues in epilepsy.Acta Neurol Belg. 2021 Jun;121(3):603-612. doi: 10.1007/s13760-020-01454-8. Epub 2020 Aug 2. Acta Neurol Belg. 2021. PMID: 32743748 Review.
-
Adjunctive Perampanel in Older Patients With Epilepsy: A Multicenter Study of Clinical Practice.Drugs Aging. 2021 Jul;38(7):603-610. doi: 10.1007/s40266-021-00865-3. Epub 2021 Jun 2. Drugs Aging. 2021. PMID: 34075567 Free PMC article.
References
-
- French JA. Refractory epilepsy: clinical overview. Epilepsia 2007;48:3–7. - PubMed
-
- Perucca E, French JA, Bialer M. Development of new antiepileptic drugs: challenges, incentives, and recent advances. Lancet Neurol 2007;6:793–804. - PubMed
-
- Rektor I. Perampanel, a novel, non-competitive, selective AMPA receptor antagonist as adjunctive therapy for treatment-resistance partial-onset seizures. Expert Opin Pharmacother 2013;14:225–235. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
